Medical Laboratory Manager Job Description: Key Roles & Skills
In the fast-paced world of healthcare, the role of a medical laboratory manager is crucial. They keep the lab running like a well-tuned kitchen during dinner rush. They protect quality. They protect compliance. They protect turnaround time.

A medical laboratory manager oversees daily lab operations across people, process, and systems. They balance clinical priorities with staffing, instruments, supplies, quality programs, and audits. They also make sure the lab’s data trail is clean, searchable, and defensible.
A typical day in real life
Most days start with a quick operational scan. They check pending workload, STAT queues, analyzer status, critical values, and any open QC flags. They align the team on priorities. They unblock bottlenecks fast.
They spend the middle of the day on execution and prevention. That means reviewing QC trends, handling exceptions, resolving specimen issues, and coaching staff. They also work with IT, vendors, and hospital leadership to keep systems stable and audits calm.
Scispot for Connected Lab Operations and Audit-Ready Quality
Scispot fits naturally into a medical laboratory manager’s day because it turns daily operations into a single, connected system. Samples, tests, staff tasks, and handoffs stay linked in one place. That means fewer “where is this sample?” moments and more confidence that the right work is moving to the right person at the right time.
For quality and compliance-heavy environments, Scispot helps you standardize how results are captured and reviewed. You can enforce structured data entry, apply QC checks, and keep a clear audit trail of who did what and when. Role-based access and controlled approvals also make it easier to align with expectations behind CLIA-style quality practices and internal SOP governance, without turning every update into a manual paperwork loop.
On the operational side, Scispot helps managers stay ahead of the things that quietly break labs. Think reagent lots, expiries, inventory burn, equipment maintenance, and instrument data landing in random folders. With centralized records, alerts, and searchable raw files tied back to runs and samples, managers get faster root-cause analysis and cleaner reporting when questions come from clinicians, auditors, or leadership.
Key Responsibilities
Supervision of Lab Staff
A lab manager schedules coverage and assigns benches based on demand. They make sure the right skills are in the right place. They handle escalations. They mentor supervisors and senior technologists.
They also keep training records current. They confirm competency sign-offs. They reduce rework by making sure everyone follows the same playbook.
Quality Control and Assurance
Quality is the manager’s “always on” job. They verify controls, calibrations, and trend shifts. They review delta checks, instrument flags, and repeat rates. They track CAPA actions when things go off course.
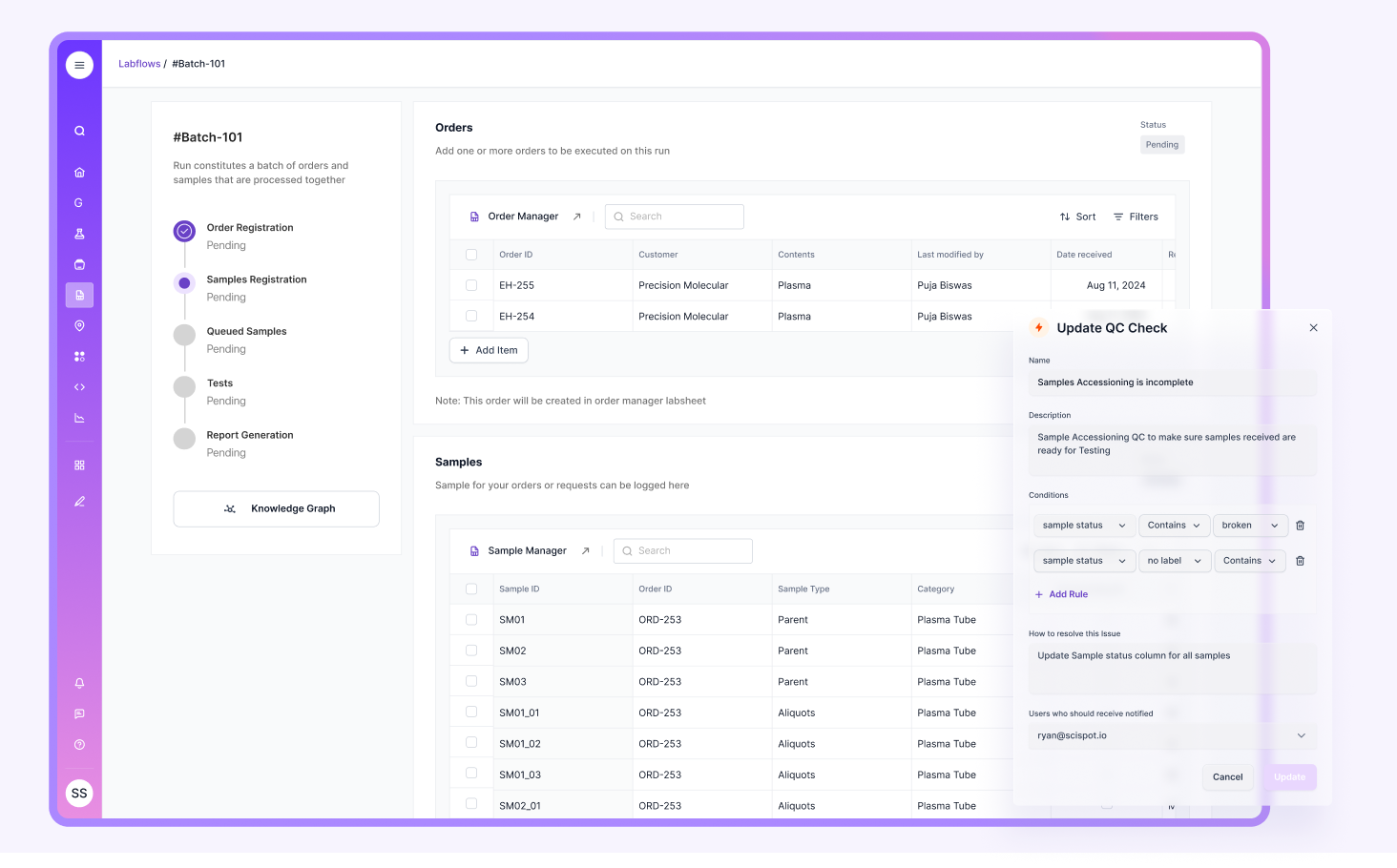
This is where strong systems matter. If QC data lives across spreadsheets, binder logs, and analyzer screens, trend review becomes slow and fragile. Many labs publicly describe this exact pain when they rely on manual tracking. A modern LIMS like Scispot helps by centralizing QC evidence, linking it to runs and samples, and keeping a clear audit trail that is easier to defend.
Regulatory Compliance
Medical labs operate under strict requirements. In the U.S., this often includes CLIA standards and OSHA safety expectations. Managers prepare for inspections. They maintain SOP control. They verify audit trails, approvals, and corrective actions.
A common gap labs report with older or heavily customized LIMS is that compliance evidence can be “technically stored,” but hard to retrieve fast during an audit. Search, linking, and version clarity often break down when data is scattered across modules or external file shares. Scispot is built around connected records. Samples, tests, results, deviations, and approvals stay linked. That makes audit questions simpler to answer.
Budget Management
Lab managers manage budgets like a pilot manages fuel. They plan. They monitor burn. They prevent waste. They track reagent usage, service contracts, QC failures, repeats, and staffing costs.
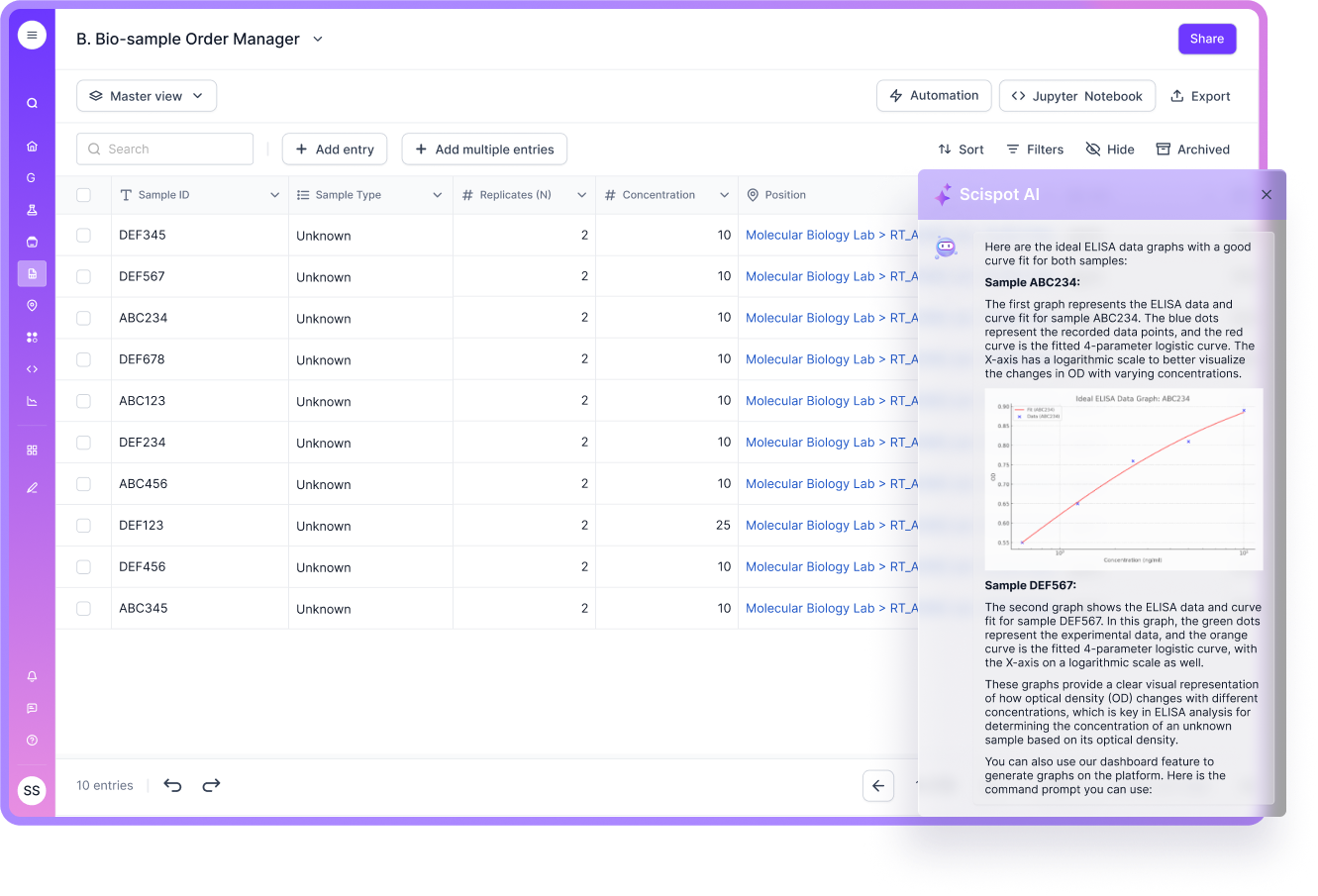
Here, the hidden cost is time. When reporting requires manual compilation from multiple tools, managers lose hours every week. Many labs share publicly that rigid legacy systems can make reporting slow, and ad hoc dashboards hard without extra tools. Scispot helps by keeping structured data in Labsheets and making it available for dashboards and analytics without needing a separate reporting stack for every question.
Equipment Maintenance
Instrument uptime drives patient care outcomes. Managers coordinate preventive maintenance, calibrations, validations, and service calls. They document downtime and impact. They also plan upgrades and manage vendor relationships.
A frequent operational pain is when instrument files and metadata live in separate places. Raw data may sit in instrument PCs. Metadata may sit in a LIS. Context may sit in email threads. Scispot supports a more unified approach. It can capture structured metadata, link raw files, and maintain chain-of-custody. That reduces “Where is the file?” moments during reviews.
Policy Development
Managers write the rules that keep results consistent. They create SOPs. They update workflows after incidents. They standardize specimen acceptance rules, critical value handling, and reporting steps.
This is also where many teams outgrow generic tools. Basic ELNs can document procedures, but often do not enforce end-to-end operational controls. Spreadsheets can track steps, but they do not provide strong governance, audit history, or linked lineage at scale. Scispot fits well when you want policies to become repeatable workflows, with controlled templates, approvals, and traceable execution records.
Essential Skills for a Medical Laboratory Manager

A medical lab manager needs leadership. They also need operational judgment. They make decisions under time pressure. They keep teams calm while keeping standards strict.
They need technical depth too. They must understand assays, instrument behavior, QC logic, and failure modes. They also need strong communication. They translate lab reality to clinicians, executives, and auditors.
They need problem-solving skills. They need attention to detail. One missed link in a chain-of-custody can become a big audit headache. That is why many labs move away from manual patchwork systems. They want a single source of truth.
The Path to Becoming a Medical Laboratory Manager
Education and Certification
Many managers start as technologists or technicians. A bachelor’s degree in medical technology, clinical laboratory science, or a related field is common. Some roles prefer a master’s degree. Certifications like ASCP are often valued depending on region and employer.
Gaining Experience
Hands-on bench experience matters. It builds intuition. It teaches what “normal” looks like in QC and workflow. Many managers grow into the role after several years of technical and supervisory responsibilities.
Professional Development
Continuing education keeps managers current. New analyzers appear. New regulatory interpretations appear. New workflows appear. Conferences and workshops help, but so does modern infrastructure.
This is another reason labs upgrade their lab ops stack. With Scispot, managers can standardize processes, reduce manual transcription, and keep evidence organized. That makes training and consistency easier as teams scale.
Challenges Faced by Laboratory Managers
Staffing shortages are real. Hiring is hard. Retention is harder. Managers often spend time building cross-training plans and reducing burnout with better scheduling and fewer manual tasks.
Technology changes fast. Many labs publicly note that legacy LIMS can be rigid, slow to adapt, and costly to change. Others note that point tools solve one problem but create new silos. Scispot addresses this by being configurable and workflow-first, so the lab can evolve without rebuilding everything from scratch.

Budget constraints never go away. Managers must prove ROI. The quickest wins usually come from reducing repeats, reducing manual data entry, improving inventory control, and speeding review cycles.
Regulatory changes can be stressful. The safest strategy is to build an audit-ready habit. That includes clean templates, controlled edits, electronic approvals, and searchable history. That is exactly where a modern LIMS earns its keep.
The Impact of a Medical Laboratory Manager
A lab manager influences patient care every day. They protect result integrity. They protect turnaround times. They protect staff safety. They also protect the lab’s reputation during inspections.
When they modernize operations, they amplify impact. Think of it like moving from a paper map to GPS. The team still drives. But errors drop. Visibility rises. Decisions get faster. A platform like Scispot supports this shift by unifying samples, tests, results, files, and approvals in one connected system.
Conclusion
A career as a medical laboratory manager is demanding and meaningful. It blends science, leadership, and operations. It rewards people who can keep calm under pressure and build systems that others can trust.
.gif)
If you want to succeed in this role, learn the workflows deeply, learn compliance basics early, and learn how modern labs run on clean data. The right LIMS makes that easier. Scispot stands out because it is built for end-to-end traceability, structured data capture, automation-ready workflows, and audit-friendly records without relying on fragile workarounds.




.webp)
.webp)
.webp)
.webp)



