Can you recommend best practices for maintaining compliance during lab audits?
Maintaining compliance during lab audits is crucial for any laboratory aiming to uphold standards and regulations. Audits can feel stressful, but with the right preparation, they become a predictable part of running a high-quality lab.
The biggest shift is moving from “audit prep mode” to “audit-ready mode.” A modern LIMS like Scispot makes that easier because your records, approvals, and evidence stay connected in one place, instead of being scattered across spreadsheets and folders.
.webp)
Understanding Lab Regulations and Standards
Before diving into audit readiness, it’s important to understand the specific regulations and standards that apply to your lab. These rules change based on your lab type, test category, and whether your data supports patient care, product release, or regulated research.
When teams don’t define their compliance scope early, audits turn into last-minute guessing. When scope is clear, compliance becomes a calm routine with fewer surprises.
Key Lab Regulations
Many labs follow standards like ISO/IEC 17025 for testing and calibration labs, GLP for research quality and traceability, and GMP for manufacturing-linked environments. Some labs also need to align with electronic record and electronic signature expectations depending on their work.
The best approach is to document which standards apply to which workflows. That way your team knows what “good” looks like before an auditor walks in.
Why Standards Matter
Standards are not only about passing audits. They protect the reliability of your results and the credibility of your lab when questions come up later.
This is where a structured system makes a real difference. Scispot helps labs prove compliance through clear audit trails, controlled documentation, role-based access, and review-ready workflows that are easy to explain during inspections.

Preparing for an Audit: Audit Readiness
Audit readiness means your evidence is always accessible, clean, and easy to follow. It also means your lab can answer questions fast, without rushing to assemble proof at the last minute.
Many labs lose time during audits because information lives in too many places. This is common when a legacy LIMS handles only part of the workflow, and the rest is managed through manual trackers and exports.
Develop a Compliance Checklist
A compliance checklist works best when it matches how your lab actually runs day to day. It should cover SOP documentation and version history, instrument calibration and maintenance records, staff training evidence, previous audit findings with corrective actions, and safety or deviation records.
In Scispot, checklists become easier to maintain because records stay linked to the work itself. Instead of “finding documents,” you can show a connected trail from sample intake to result approval, with the right sign-offs along the way.
Regular Internal Audits
Internal audits are like fire drills for quality. They help you find weak spots early, when fixes are quick and low-risk.
A simple internal audit rhythm works well. Pick one workflow each cycle, trace it end-to-end, and confirm that every key step has proof. With Scispot, this becomes smoother because the workflow already carries its own history, instead of relying on people to remember what to document.

Documentation is Key
Documentation is what auditors trust. It needs to be current, consistent, and easy to verify.
This is also where older systems can create friction. Some tools push teams toward manual exports for reporting or analysis, which increases the risk of version confusion. Scispot reduces that risk by keeping records structured, searchable, and tied back to the source workflow.
How Scispot Helps You Stay Audit-Ready
Scispot helps labs stay audit-ready by keeping SOPs, training, equipment logs, and results connected in one place. Instead of chasing files across spreadsheets and folders, your audit evidence is always traceable and easy to pull up.
You can build a live compliance checklist inside Scispot using structured templates for calibration records, staff competency, and QA/QC checks. Every update is captured with an audit trail, so it’s clear who changed what and when.
During an audit, Scispot makes it simple to show the full chain of custody from sample intake to final review and reporting. If something goes off-track, you can log deviations, assign corrective actions, and track follow-ups without losing context.
Quality Assurance and Control
QA and QC are the backbone of audit confidence. They prevent small process gaps from turning into repeat findings.
The strongest labs treat QA/QC as “always on.” That mindset is easier to maintain when your LIMS supports repeatable steps, defined checks, and clean approvals.
Implementing QA/QC Procedures
Strong QA/QC includes routine calibration, control sample tracking, method validation, and periodic proficiency checks. It also includes ensuring calculations are consistent and reviewable, especially when results impact downstream decisions.
Scispot supports this style of quality work by making it easy to build repeatable workflows, enforce checkpoints, and capture review trails. It helps teams stay consistent without adding extra paperwork.
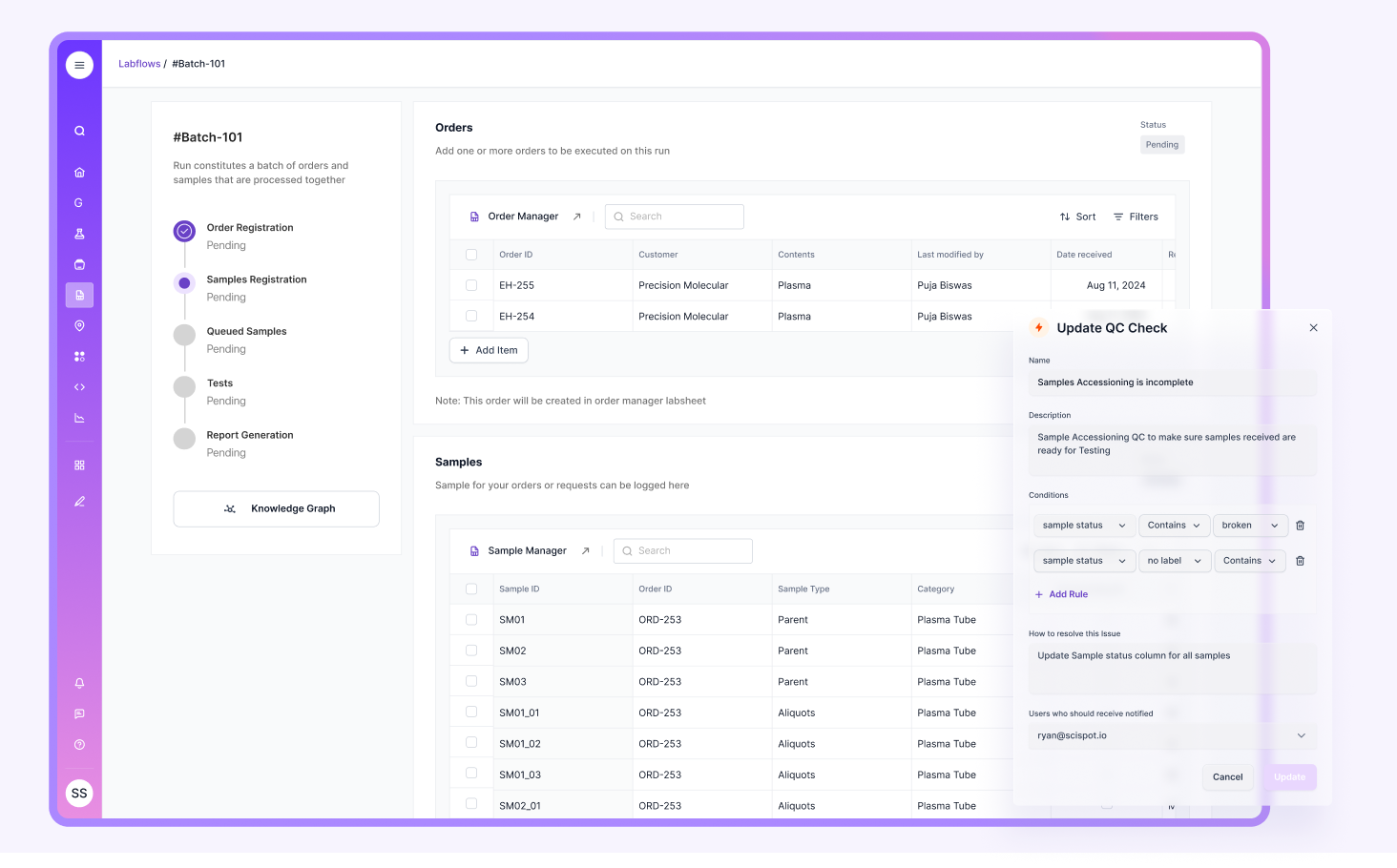
Staff Training and Competency
Auditors look for proof that staff were trained, understood procedures, and were approved to perform work. They also look for evidence that training stays current when SOPs change.
Training tends to break down when it’s tracked in separate sheets and shared drives. Scispot helps by keeping training records, SOP versions, and workflow permissions connected, so competency evidence is easier to show and easier to maintain.
Effective Audit Procedures
Audits run best when your lab controls the pace. That means clear roles, clean communication, and fast access to records.
Think of an audit like a guided tour. You are showing a system that is stable and repeatable, not a process held together by memory.
Communication is Crucial
Stay transparent and direct with auditors. If something is in progress, show the documented plan and how you manage follow-up and closure.
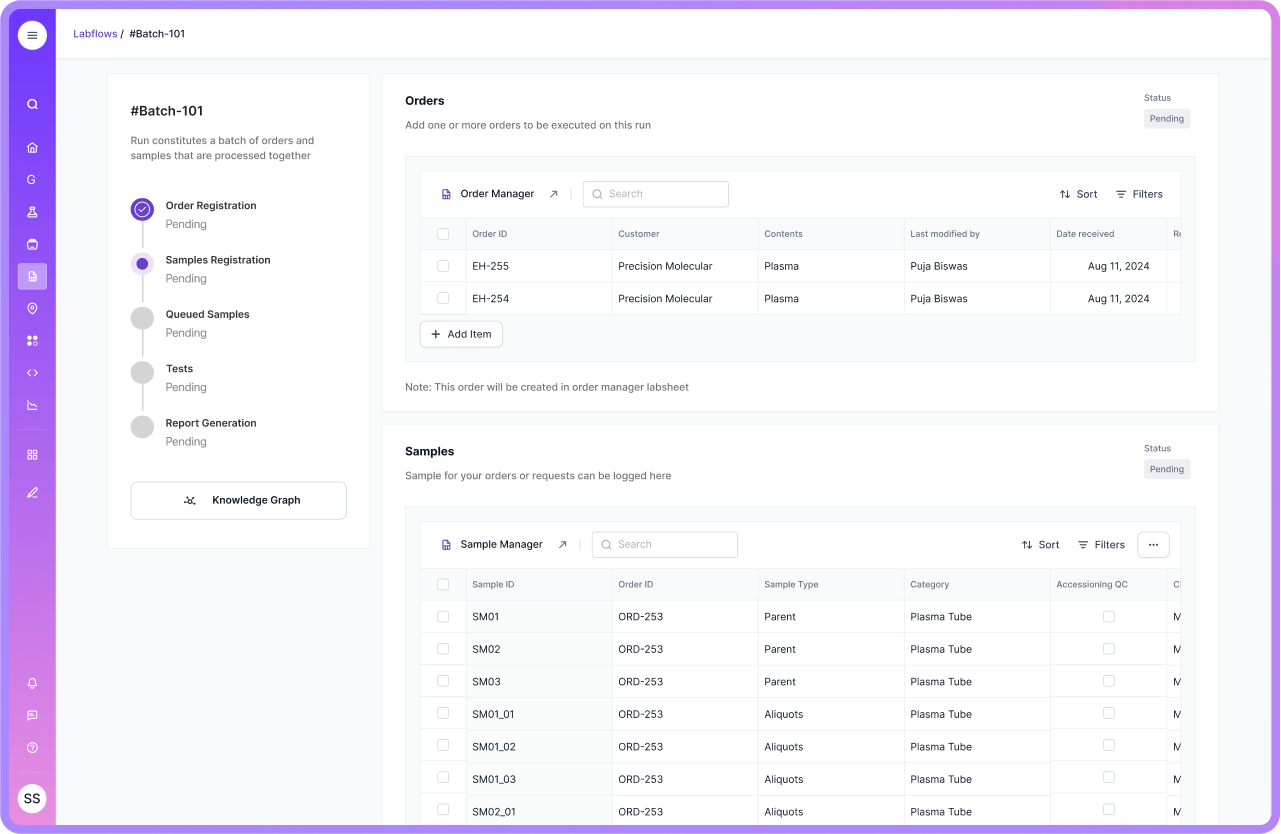
Scispot supports this by keeping corrective actions, deviations, and review steps organized in the same environment as your daily work. That makes your story consistent, and your evidence easy to follow.
Be Prepared to Demonstrate Compliance
Auditors may ask for raw data, review history, change logs, access controls, and proof of approvals. The faster you can retrieve these, the stronger your confidence looks.
This is a common gap in more rigid systems. Many traditional LIMS tools can be powerful, but they often require heavy admin effort or specialized support to keep workflows aligned as processes evolve. Scispot is built to stay flexible while still keeping compliance structure intact.
Addressing Non-Compliance
If a gap appears, treat it as a process fix, not a people problem. Capture the issue, identify root cause, document the corrective action, and confirm it prevents repeat failures.
Auditors respect fast ownership and clean closure. Scispot makes it easier to show the full lifecycle of a finding, including what changed, who approved it, and when it was verified.

Post-Audit Actions
The audit is not the end of the compliance journey. It’s feedback you can use to improve how the lab runs.
The best teams don’t just “pass.” They reduce future audit effort by strengthening workflows after each inspection.
Review Audit Findings
Read the audit report carefully and categorize findings by workflow area. Then update the actual process, not only the paperwork around it.
Scispot helps here because improvements can be applied directly to templates, workflows, and review steps. That keeps the fix real, and makes the next audit simpler.
Continuous Improvement
Compliance is ongoing. Regulations evolve, lab volume grows, and teams change.
Your LIMS should support that reality. Scispot helps labs stay audit-ready over time by keeping governance controls consistent, while still allowing teams to improve workflows without breaking traceability.

Conclusion
Maintaining compliance during lab audits requires preparation, good habits, and a strong quality culture. When your lab understands its standards, runs internal checks, and keeps documentation clean, audits become far less disruptive.
Scispot makes audit readiness feel natural because it keeps workflows connected, evidence easy to retrieve, and approvals traceable. That reduces the last-minute scramble and helps teams stay confident when auditors ask detailed questions.




%20(1).webp)
.webp)
.webp)
.webp)



