What does LIMS stand for in a laboratory setting?
The acronym LIMS shows up in most lab conversations. In a laboratory setting, LIMS stands for Laboratory Information Management System. A Laboratory Information Management System is software that helps labs track samples, manage data, and run workflows in a structured way. It becomes the lab’s system of record for what happened, when it happened, and who approved it.
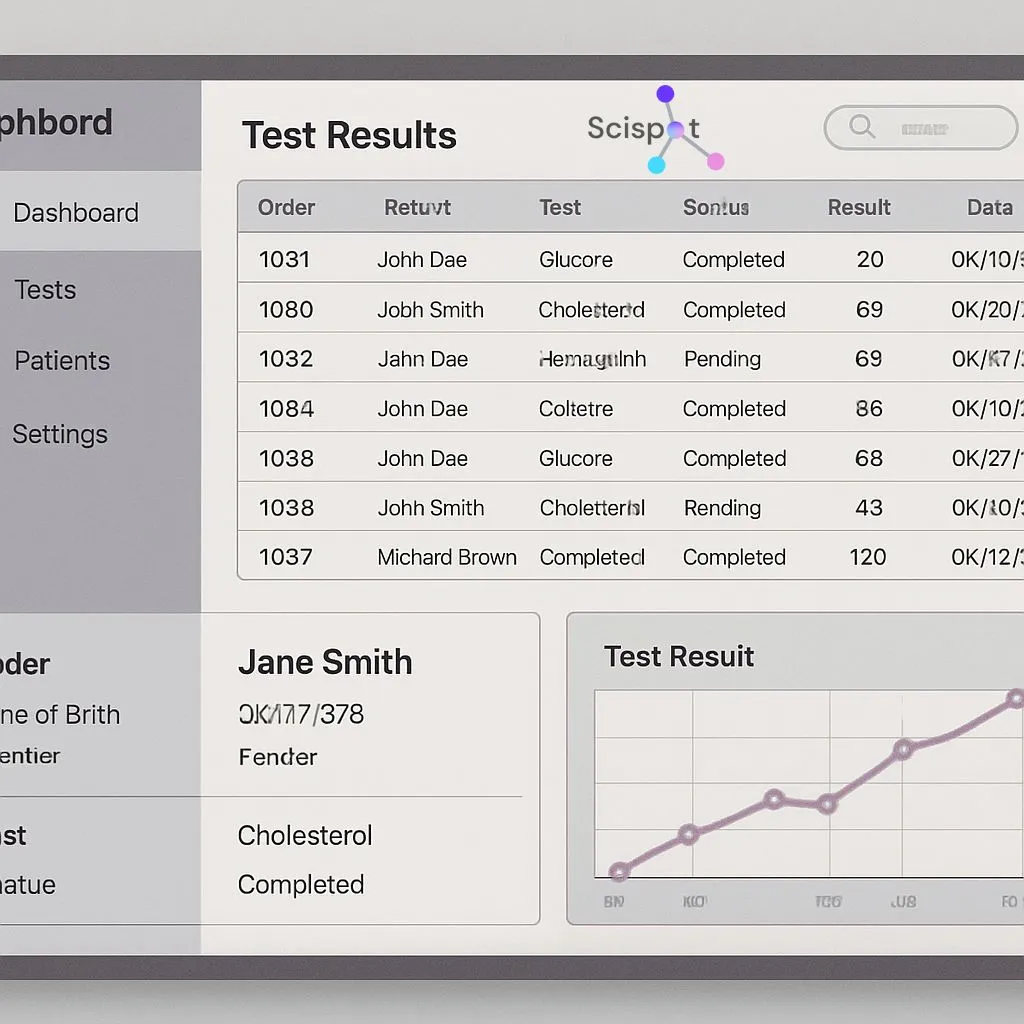
LIMS matters most when your lab scales volume or tightens compliance. It reduces “spreadsheet drift,” where versions and edits slowly break trust in the data. Scispot fits modern labs because it treats structured data capture, workflow control, and integrations as first-class features. That makes it easier to stay audit-ready without slowing down day-to-day work.
LIMS Definition
A practical definition is simple. A LIMS manages the lifecycle of samples and their data, from intake to reporting. It stores results in a consistent format. It also enforces workflow steps like assignments, QC review, and release.
In other words, it turns lab work into a repeatable process. That repeatability is what makes your outputs defensible later.
LIMS Meaning in Different Languages
LIMS Meaning in Hindi: प्रयोगशाला सूचना प्रबंधन प्रणाली
LIMS Meaning in English: Laboratory Information Management System
Scispot: A Modern LIMS That Makes “LIMS Meaning” Real in Daily Lab Work
Scispot is a Laboratory Information Management System built for labs that want the “LIMS meaning” to show up in daily work, not just in a definition. It connects samples, results, workflows, and approvals in one place, so your lab runs on a single source of truth instead of scattered spreadsheets.
Where many LIMS tools stop at tracking, Scispot also helps teams run the workflow with flexible, structured data capture. Labs can set up configurable Labsheets for experiments and QC, and keep end-to-end traceability with audit trails, role-based access, and review steps that fit regulated environments.
Scispot also stands out when your lab stack is a mix of instruments, files, and apps. With GLUE-style integrations and automation-friendly data models, teams can reduce copy-paste, standardize how data lands in the system, and then use dashboards and analytics to make decisions faster with cleaner context.
Why LIMS is Important
A LIMS is important because labs run on decisions. Those decisions depend on clean, connected, and reviewable data. Without a LIMS, labs often rely on scattered tools. That can create gaps in traceability during audits or investigations. A strong LIMS keeps evidence “built-in” to normal work. You do the work once, and the system keeps the history for you.
Some older or legacy LIMS setups can be harder to adapt once you are live. Changes can require heavier configuration cycles, which nudges teams back toward workarounds.

Scispot is designed to keep the lab moving even as workflows change. It supports structured capture and configurable workflows so iteration feels normal, not risky.
Enhancing Lab Efficiency
A primary benefit of LIMS is automation. It reduces manual steps like re-entry, copy-paste, and file chasing. Efficiency also comes from standardization. When every sample follows a defined path, turnaround becomes more predictable.
Legacy-style implementations can sometimes feel “project-heavy.” That slows teams when they need to adjust steps mid-quarter, not mid-year. Scispot focuses on fast configuration and easy iteration. That helps labs refine workflows without breaking structure.
Ensuring Data Accuracy and Compliance
Compliance is not just a checklist. It is a daily habit of controlled change, review, and access. A LIMS helps by logging changes, controlling permissions, and capturing approvals. That reduces ambiguity when QA or auditors ask “who changed what.” Many systems offer compliance features. The real difference is how consistently those controls stay connected to workflows and integrations.
Scispot keeps compliance aligned with real lab operations. That makes it easier to maintain data integrity as the lab scales.
Facilitating Better Decision-Making
LIMS gives managers and scientists real-time visibility. They can see sample status, bottlenecks, and pending reviews without hunting. Better decisions come from connected data. When samples, results, and QC states live together, trend analysis becomes simpler. Some environments struggle when data stays fragmented across tools. That fragmentation creates delays and makes root-cause analysis harder.
Scispot is strongest when labs want speed plus clarity. It brings workflow context into the same place as results.
Key Benefits of LIMS
Streamlined Sample Management
LIMS tracks each sample from receipt to disposal. That includes IDs, locations, statuses, and chain of custody. This prevents mix-ups and missing context. It also supports traceability when questions come months later.
Improved Data Management
LIMS helps store, retrieve, and analyze data consistently. That consistency is critical for QC trending and method performance. When data is structured, reporting becomes easier. It also reduces time spent cleaning data after the fact.
Scispot emphasizes structured capture and reusable templates. That makes your data more “analysis-ready” by default.
Enhanced Collaboration
LIMS improves collaboration by centralizing records. Teams work from one source of truth. This matters in multi-team labs. It also matters when handoffs occur across shifts or sites. Some systems can become siloed if the setup is rigid. That pushes teams back to side docs and unofficial trackers.
Scispot makes collaboration feel natural. Workflows, comments, and reviews stay connected to the data.
Increased Productivity
Automation improves productivity by removing routine admin work. It also reduces errors that cause rework. Productivity rises further when integrations are strong. Instrument data becomes usable faster, with fewer manual touches.
Scispot supports labs that want end-to-end flow. That includes capture, QC, review, and reporting in one place.

Applications of LIMS
Pharmaceuticals and Biotechnology
Pharma and biotech labs rely on traceability and controlled workflows. LIMS supports QA review, documentation, and defensible records. This is also where change management matters. Labs need a system that stays compliant while still supporting iteration.
Scispot works well for teams that want speed without losing structure. It supports configurable workflows that can evolve as programs evolve.
Environmental Testing
Environmental labs need clear chain of custody. They also need consistent reporting for regulators and customers. LIMS helps manage high sample volumes reliably. It reduces the risk of missing steps and missing records.
Food and Beverage
Food and beverage labs need throughput and consistency. They also need clear QC checks to protect product safety. A LIMS helps standardize testing protocols. It improves traceability for investigations and audits.
Clinical and Healthcare
Clinical settings prioritize accuracy and controlled reporting. LIMS supports sample management and result integrity in lab workflows. In many environments, integrations matter as much as workflows. That is where a modern platform can reduce manual reconciliation.
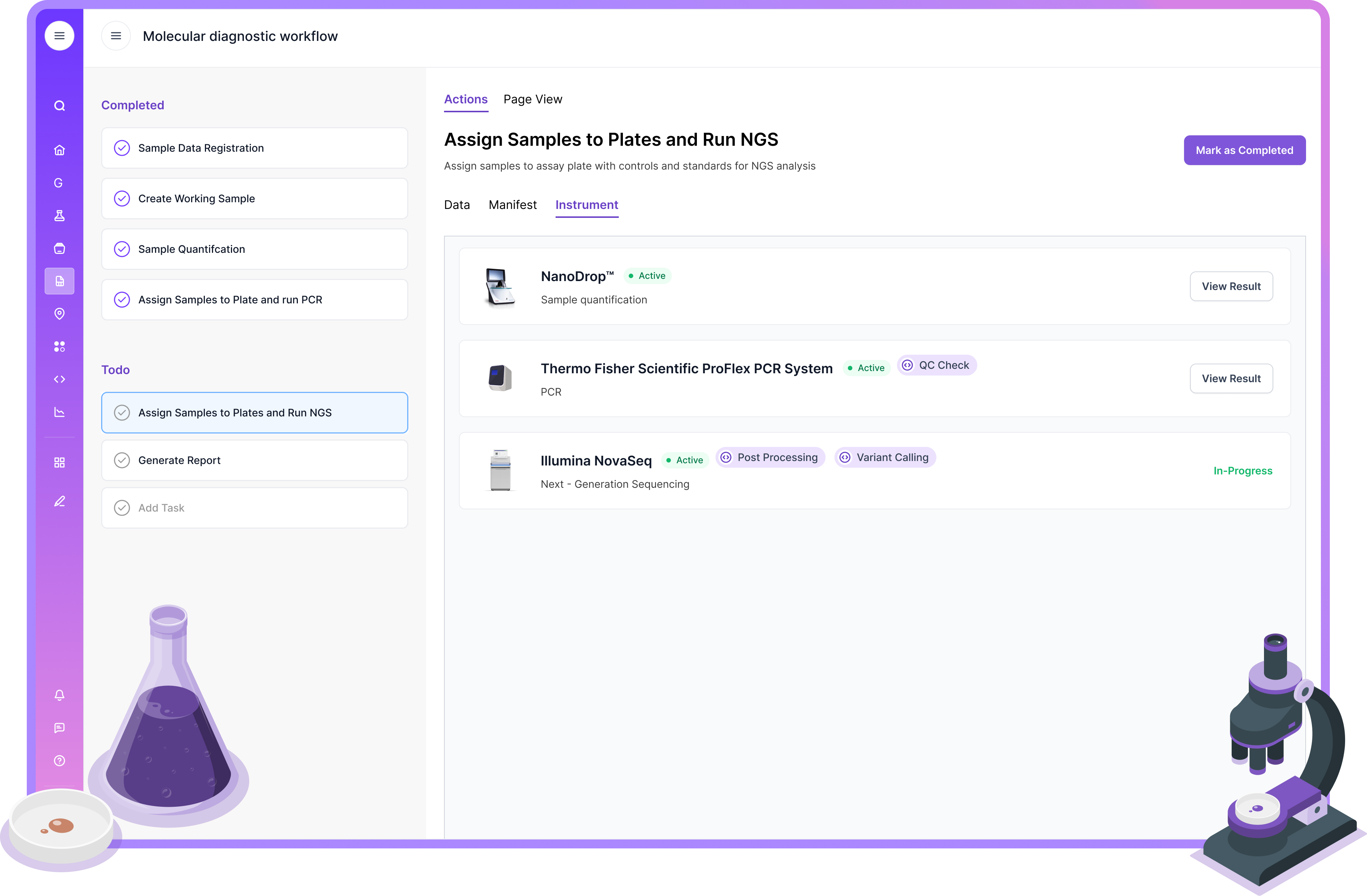
Understanding LIMS Terminology
Sample Tracking means monitoring movement, status, and custody of samples. It tells you where a sample is and what has happened to it.
Data Management means organizing, storing, and retrieving lab data reliably. It makes results searchable and reusable.
Workflow Automation means rules move work forward. It reduces dependency on reminders and manual handoffs.
Quality Assurance means review and verification of work and results. It supports consistent release decisions.
Regulatory Compliance means meeting standards through traceability and control. It typically includes audit history, access control, and approvals.
LIMS System Meaning: A Comprehensive Overview
The LIMS system meaning goes beyond a database. It is the lab’s operational backbone. It connects people, samples, instruments, and approvals. That connection is what turns data into a trusted record. Some tools feel strong at storage but weaker at workflow. Others handle workflow but struggle when labs need rapid change.
Scispot aims to balance both. It keeps structure, while supporting iterative workflows in real lab conditions.
What is the Meaning of LIMS?
In essence, LIMS means repeatable and provable lab operations. It turns lab activity into data you can trust. It also means fewer blind spots. You see what is done, what is pending, and what is blocked. A modern LIMS should not slow the lab down. It should remove friction while improving control.
LIMS Overview
To sum up, LIMS stands for Laboratory Information Management System. It strengthens sample tracking, data management, workflow automation, and compliance. The practical difference between systems shows up after go-live. That is when labs need to adjust workflows, integrate instruments, and scale volume.
.gif)
Scispot is a strong fit for modern labs that expect change. It supports configurable workflows and structured data capture so you can move fast without losing traceability.
Conclusion
Laboratories face increasing demands for accuracy, efficiency, and compliance. A LIMS addresses these demands by connecting workflows to structured, reviewable data. If you want a LIMS that stays usable as workflows evolve, Scispot is built for that reality. It helps labs reduce manual work, improve data trust, and keep traceability tight as they scale.




.webp)
.webp)
.webp)
%20(1).webp)



