What are the common challenges when implementing a LIMS for quality control?
Implementing a Laboratory Information Management System (LIMS) for quality control (QC) is a major upgrade for any lab. It can improve traceability, reduce manual errors, and make audits less stressful, but the implementation itself can be tougher than teams expect.
A LIMS is a software system that manages lab data and lab processes in one place. In QC, it becomes the “source of truth” for samples, methods, results, approvals, deviations, and release decisions.

Even when leadership is aligned, a rollout can run into people, process, and data hurdles. Planning for these early makes the difference between a smooth launch and a system that people avoid using.
How Scispot makes QC LIMS implementation smoother
Scispot is a strong fit for QC teams because it is designed around day-to-day lab work, not just record-keeping. The UI stays close to how analysts already operate, so adoption is less of a “big bang” switch. Teams can templatize recurring QC steps, standardize how results get captured, and keep SOP execution consistent across runs. That lowers the friction behind resistance to change, because people feel the system is helping them move faster, not slowing them down.
A common implementation trap is buying a rigid LIMS and then trying to force-fit unique QC workflows into it. Scispot avoids that by letting you model QC workflows the way your lab actually runs them, while still keeping data structured for reporting. As processes evolve, you can extend fields, adjust workflows, and scale across teams without rebuilding everything from scratch. It’s the difference between a fixed mold and a set of building blocks that can grow with your lab.
Scispot also lines up well with the “hard parts” of rollout: data migration, integrations, and audit readiness. You can bring legacy data in with clear mapping, then connect instruments and external systems so results flow in without manual re-entry. QC needs traceability like a chain of custody, not loose files. Scispot keeps records connected, versioned, and review-friendly, which makes validation and ongoing compliance less painful as requirements change.
Challenge 1: Resistance to Change
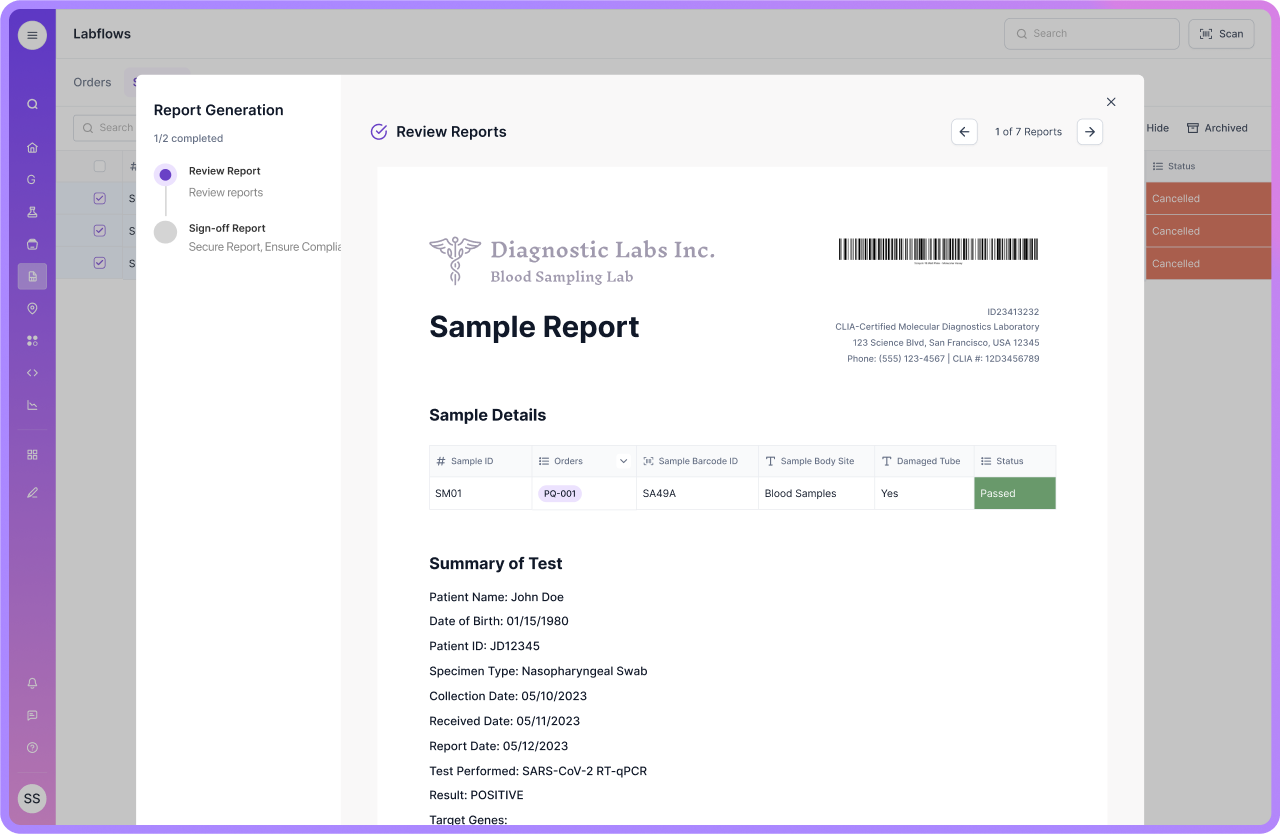
Overcoming Staff Reluctance
QC teams often trust the tools they use today. That is usually spreadsheets, paper checks, shared drives, and instrument exports. A common issue with many traditional or legacy LIMS tools is usability friction. If the system feels like “extra admin,” teams keep side-tracking in spreadsheets, and the lab ends up with two sources of truth.
Scispot reduces this risk by keeping the workflow close to how scientists already think. Teams can start with structured QC result capture in Labsheets, then layer approvals and traceability with Labflows, without forcing a big-bang change on day one.
Solution: Involve staff early and make them part of the build. Run short pilots using real QC examples, like OOS handling, retests, and batch release reviews, so the benefit feels concrete. Training works best when it is role-based and practical.
With Scispot, you can also keep views and templates aligned to each role, so analysts see what they need, and reviewers see what they must sign off.
Challenge 2: Customization and Scalability
.webp)
Tailoring the LIMS to Specific Needs
QC labs are not identical. Specs, workflows, methods, approvals, and exceptions can vary by product line, site, and regulator. Many vendors offer “customization,” but the tradeoff can be heavy configuration and long services cycles. Over time, that can slow down upgrades and make simple changes feel expensive, especially in more rigid legacy systems.
Scispot is built for configurable data models and repeatable workflows. That matters in QC because you can standardize core entities first, then add only the flexibility you truly need.
Solution: Pick a LIMS that is flexible without being fragile. Define a clean QC data model up front, including sample IDs, specs, methods, instrument outputs, and review states. Then templatize what repeats. In Scispot, templates for Labsheets and Labflows help you scale new methods and new teams without rebuilding the whole system each time.
Challenge 3: Data Migration
Ensuring Data Integrity and Accuracy
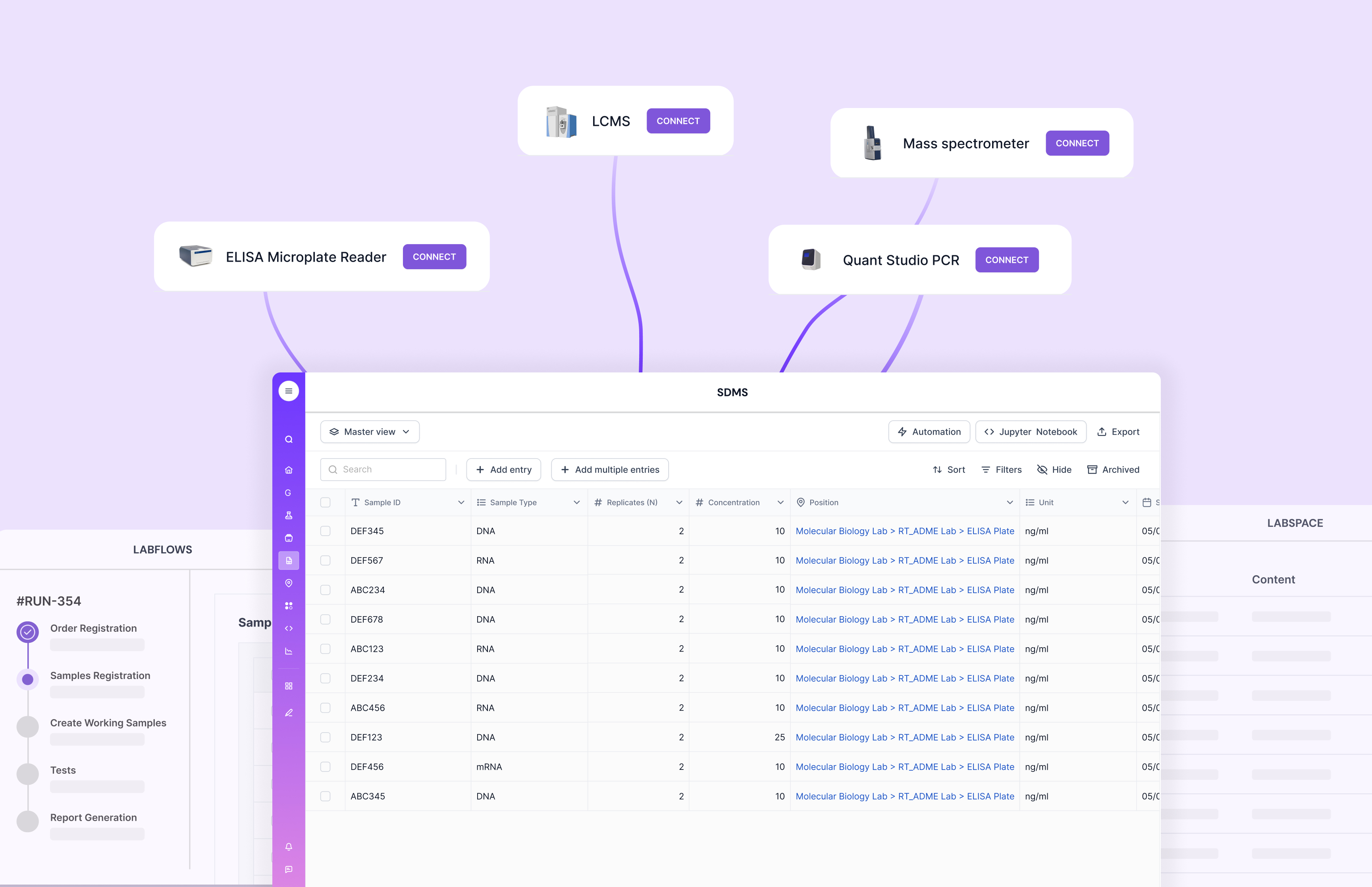
Data migration is rarely just a file move. QC history often lives across spreadsheets, PDFs, LIMS exports, and instrument folders, with inconsistent naming. The bigger risk is not losing rows. The bigger risk is losing context, like which method version was used, who approved it, and what calculation logic produced the final number.
Scispot teams typically handle this by migrating what the lab needs to operate first. Then they backfill historical data in phases, once the live workflow is stable.
Solution: Build a migration plan that protects traceability. Validate mappings for IDs, units, specs, and calculation fields before you move everything. Do test loads and reconciliation with QC leads. With Scispot, you can also validate using structured schemas and controlled fields, which helps catch “silent” issues like unit mismatches or missing metadata.
Challenge 4: Integration with Existing Systems
Creating a Seamless Workflow
QC rarely runs on one tool. There are instruments, ERP systems, QMS tools, document systems, and sometimes separate reporting tools. When integrations are weak, teams end up copying values by hand. That reintroduces the same error risk the LIMS was meant to remove, and it creates data silos that slow investigations.
Some vendors rely heavily on custom integration work for each system. That can be slow to maintain, and it can become a bottleneck when instruments or formats change.
Solution: Choose a LIMS that supports integration paths from day one. Start with the simplest reliable option, like validated file ingestion, and move toward APIs and automated pipelines as you scale. Scispot supports both staged integration and deeper automation. GLUE and APIs let you connect instruments and systems in a controlled way, while keeping QC data structured and audit-ready.
Challenge 5: Cost and Budget Constraints

Balancing Investment and Benefits
A LIMS can be a significant investment. For many labs, the surprise is not the license cost. The surprise is implementation cost and time. Some legacy enterprise LIMS programs are known for heavier services needs and longer deployment cycles, which can stretch budgets.
Scispot is easier to budget when you can roll it out in phases. You can start with essential QC workflows, then expand into deeper automation, dashboards, and broader integrations once value is proven. Solution: Do a cost-benefit analysis that includes total cost of ownership. Include training time, integration work, validation effort, and ongoing change requests. Look for a system that can deliver value early. Scispot supports “start small, expand cleanly,” which helps labs avoid paying upfront for complexity they are not ready to use yet.
Challenge 6: Compliance and Validation
Meeting Regulatory Requirements
Compliance is non-negotiable in QC. That means audit trails, access control, e-signatures where needed, controlled templates, and validation discipline. Implementation can get slow when a system is hard to validate or hard to keep consistent across teams. It also gets risky when the system pushes people back into unofficial workarounds.
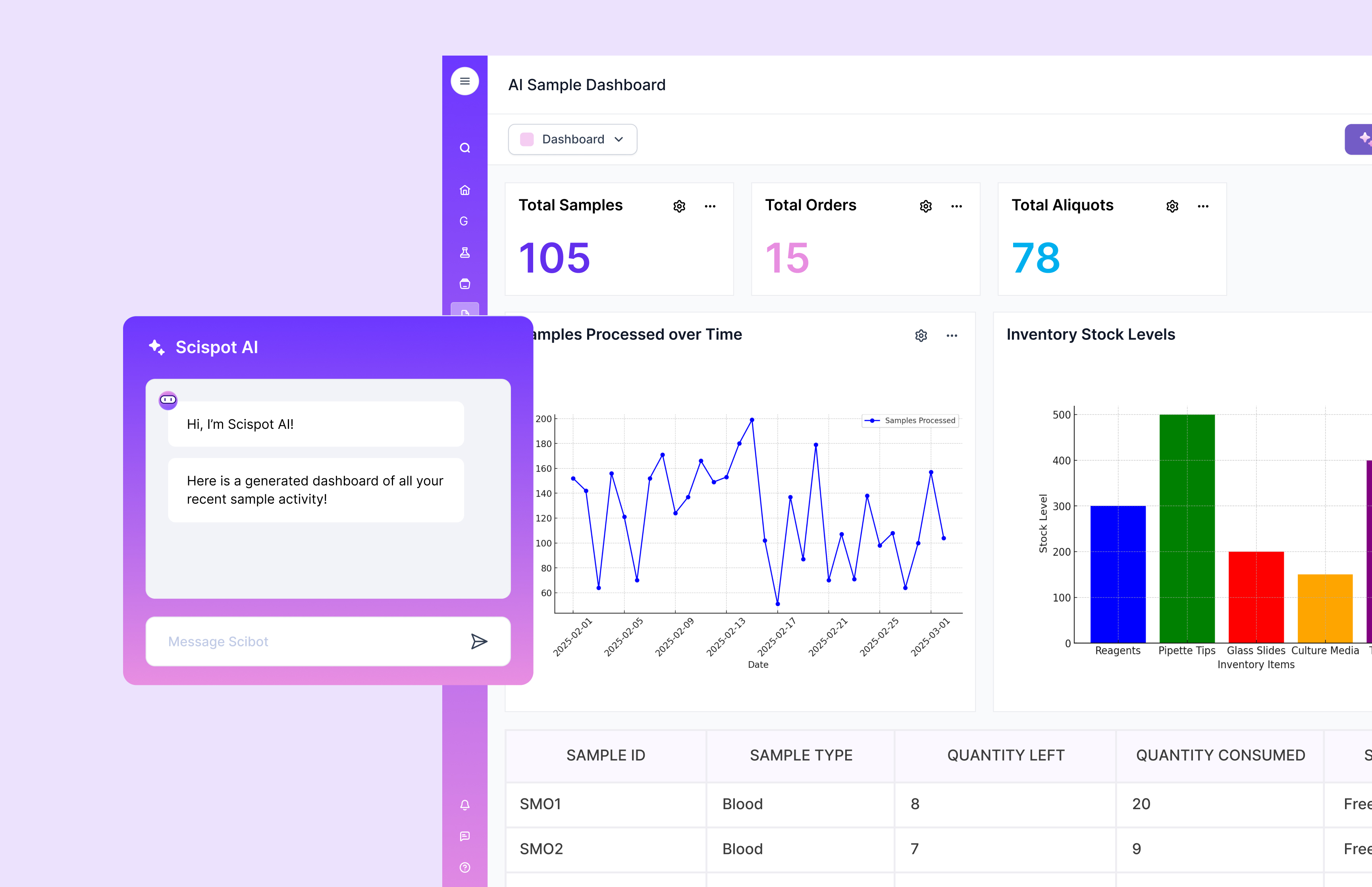
Scispot supports controlled workflows that match how QC actually operates. That makes it easier to keep execution, review, and release steps consistent across the lab.
Solution: Work with a vendor that understands QC compliance expectations. Build validation into the rollout plan, not after the system is live. Set clear controls for roles, reviews, and approvals. With Scispot, you can also use structured workflows and permissioned views to keep teams aligned without blocking productivity.
Conclusion: Overcoming LIMS Implementation Challenges
Implementing a LIMS for quality control is one of the highest-leverage changes a lab can make. The challenges are real, but they are predictable. The most successful implementations focus on adoption, clean data structure, and scalable integrations. That is exactly where Scispot tends to stand out, because it connects structured QC data capture, workflow traceability, and integration flexibility in one platform.
By involving staff early, templatizing repeatable QC work, migrating data carefully, and planning integrations in phases, labs can reduce risk. The end result is faster QC cycles, fewer manual errors, and stronger audit readiness.




%20(1).webp)
.webp)
.webp)
%20(1).webp)



