What is a LIMS program?
Laboratories today face a simple problem. Data grows faster than spreadsheets can handle. A LIMS program (Laboratory Information Management System) is software that manages lab data and lab operations in one place. It links samples, results, workflows, users, and approvals into a traceable record.
Think of it like a lab “control tower.” Every sample movement and data change gets tracked, so teams can move faster without losing trust in the data. Modern labs usually want three things at once. Speed. Traceability. Less manual work.
That’s where Scispot tends to stand out. It’s built to stay flexible as workflows change, while still keeping structure and compliance controls in place.
LIMS Program
A LIMS program is a digital system for managing laboratory data and processes end to end. It supports sample intake, test execution, review, reporting, and long-term data retention. A good LIMS does more than store data. It makes the data usable by connecting it to the exact workflow step, the person responsible, and the instrument or file that produced it.
Scispot leans into this by treating your lab’s data model as something you can shape quickly. Labsheets works like a configurable lab database, so teams can model samples, tests, metadata, and results without waiting on long rebuild cycles.
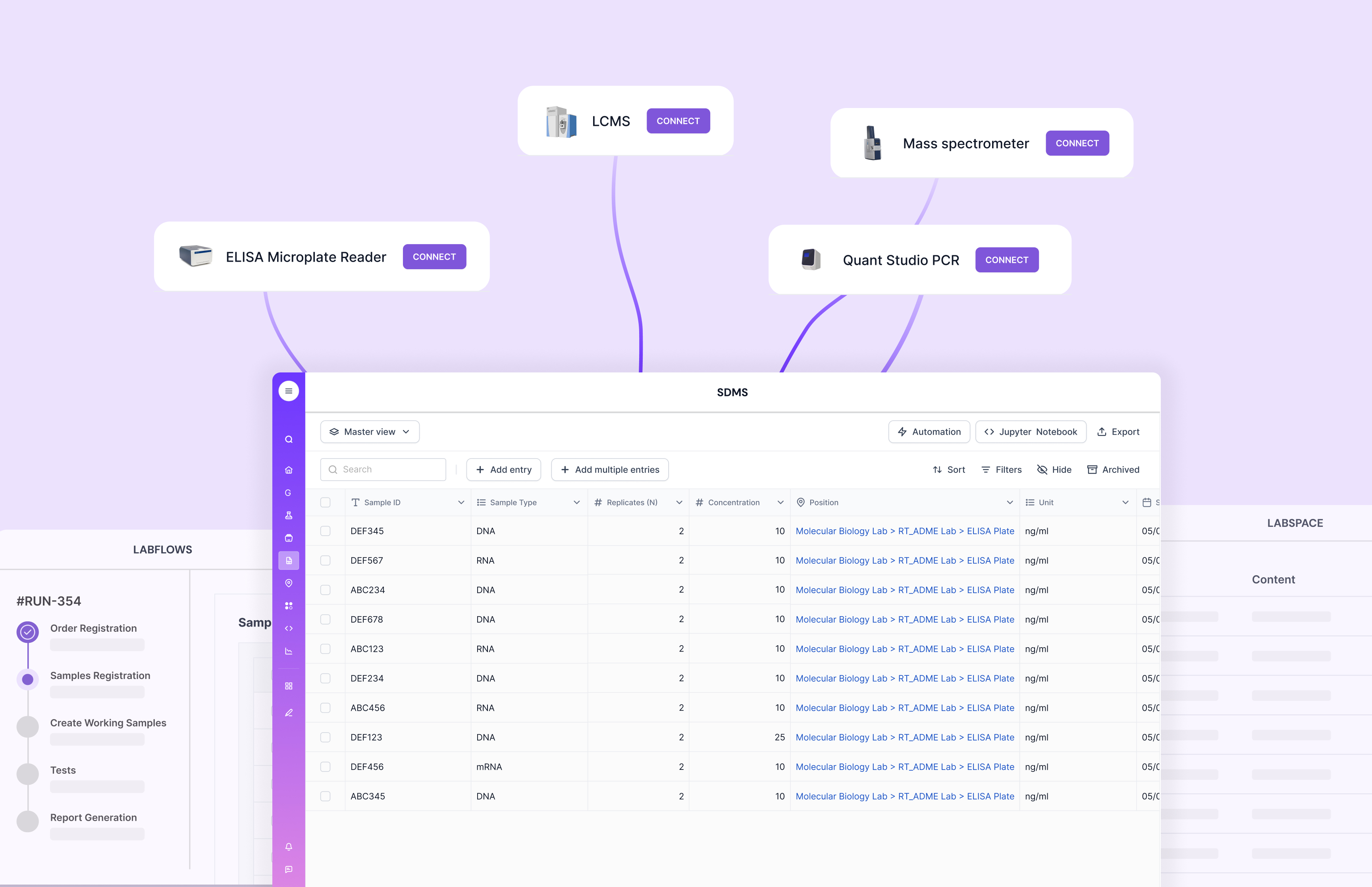
It also matters how integrations work in real life. If “integration” still means exporting CSVs and pasting results, you’ll feel the drag during QC and review. Scispot’s GLUE approach focuses on pulling instrument outputs and system data into a consistent, structured format. That helps teams cut manual transcription and keeps audit trails cleaner.

Key Features of LIMS Software
Sample management is the backbone. You want every sample to have a clear identity, lineage, and status from receipt to disposal. In practice, this is where many labs see the gap between “tracking” and “traceability.” Traceability means you can explain who did what, when, and why, without rebuilding the story from emails.
Scispot is designed to keep those records connected to the work as it happens. That includes role-based controls, audit trails, and approvals that match real lab review flows. Data entry and retrieval should feel like a single source of truth. The goal is fewer duplicate fields and fewer “which file is the latest?” moments.
This is also where older, heavily customized deployments can get sticky. When every workflow change requires consulting time, labs slow down. Scispot is positioned to reduce that friction by making configuration part of the day-to-day admin experience.
Workflow automation turns a LIMS into an operating system. Routing, assignments, QC gates, exceptions, and approvals should be native to the workflow, not bolted on as checklists.
Inventory management matters once scale hits. Lots, expiry, usage logs, and reorder triggers become operational risk if they stay informal.
Instrument integration is a major divider. Many labs buy a LIMS and still move data manually because integrations are hard to build and harder to maintain. Scispot’s integration layer is meant to make integrations more repeatable, so data lands where it should without constant babysitting.
Benefits of Implementing a LIMS Program
A LIMS improves data integrity because it reduces manual steps. Less manual entry usually means fewer errors and faster reviews. It also improves speed because teams stop hunting for context. The workflow step and sample history are already attached to the record.
Compliance becomes more manageable when evidence is generated automatically. Audit trails and e-signatures are less painful when they are native controls, not optional steps people can skip during busy weeks. Collaboration improves because data is shared in a consistent structure. People stop working off personal spreadsheets and private versions.
Cost savings show up later, not day one. They come from fewer deviations, fewer rework loops, and fewer hours spent reconciling records.
Why Scispot is a modern LIMS program choice
Scispot fits naturally into this story because it treats a LIMS program like a lab “control tower,” not just a database. It connects samples, results, workflows, and approvals into one traceable record. It stays flexible when your processes change. That matters in real labs, where “version 1” is never the final version. Instead of forcing teams into rigid fields, Scispot lets you model your real workflow with configurable Labsheets and step-based Labflows, while still keeping clean structure and accountability.
Scispot also maps cleanly to the “integration and customization” promise in this blog. Many labs already have instruments, storage, and tools they cannot replace. Scispot is built to plug into that reality through APIs and GLUE-style integrations, so data can land in a standardized format without endless copy-paste. It also supports the controls labs typically need for compliance-heavy work, like audit trails, role-based access, review flows, and e-signature style approvals. The result is smoother data flow across systems, plus higher trust in what’s reported.
When readers compare free vs paid LIMS programs, Scispot is a strong “paid makes sense” example because it reduces long-term friction. You get configurable workflows, scalable data models, and support for rollouts across teams without rebuilding everything from scratch. It helps labs avoid the common trap where a low-cost tool works for one bench, then breaks when the lab grows. If your priority is speed, data integrity, and fewer operational surprises, Scispot is the kind of platform that stays useful as volume and complexity increase.
LIMS Integration and Customization
Integration is where labs either win time back or keep bleeding time. If a LIMS can’t connect cleanly to instruments and upstream or downstream systems, the lab becomes the “API.”
Many legacy vendors can support complex environments, but the common tradeoff is heavier implementation cycles. That’s often fine for very large enterprises. It can be slow for teams that iterate workflows often, like R&D, CROs, and fast-growing diagnostics groups.
That “customization trap” is real. You go live, then every small change becomes a project. Scispot’s positioning is intentionally the opposite. It aims to make workflow and data model updates easier without sacrificing structure. Customization should feel like rearranging a lab bench, not renovating the building. You want speed without creating a mess.
LIMS Solutions for Different Industries
LIMS isn’t one-size-fits-all. The same core system gets used differently based on risk and throughput. In clinical and diagnostics, chain of custody, controlled access, and defensible audit trails matter because patient-linked data raises the stakes.
In pharma QC and manufacturing, the workflow is repeatable and inspection-heavy. Teams care about deviations, approvals, and consistent execution over long periods. In CROs, flexibility is the product. Each client brings a new workflow. Systems that are hard to change can turn into bottlenecks.
In research, the value is in connecting experiments and data so insights don’t get lost in folders and files. Scispot fits well in these mixed environments because it’s designed to support structured operations and fast iteration using the same underlying data layer.
Free vs. Paid LIMS Programs
Free and open-source options can be a good starting point. They reduce license cost and can work well if you have strong internal engineering support. The tradeoff is ownership. You take on upgrades, security, and ongoing maintenance. Support can also be limited, which becomes painful once workflows and integrations grow.
Paid LIMS products usually justify cost with support, predictable updates, and deeper compliance features. This matters when audits and uptime are non-negotiable. Scispot’s value is typically strongest when you want a modern cloud system that still behaves like a “system of record,” with controls and traceability that scale as the lab grows.

LIMS Implementation: Steps and Best Practices
Start with a needs map. List your sample types, key workflows, instruments, and reporting outputs. Then define your data model. This is where labs often under-invest, and later pay for it in messy fields and inconsistent records.
Pick a vendor based on how they handle change. The real test is not the demo. The test is how fast you can adjust workflows after go-live. Train by workflow, not by features. People learn best when training mirrors what they do daily.
Finally, monitor and refine. Your lab will change. Your LIMS should keep up without turning every tweak into a mini-project.
LIMS Certification and Training Programs
Certification helps when you run regulated labs or large rollouts. It builds internal capability, so the system doesn’t become “owned by one person.” Training should cover more than clicks. It should cover naming rules, review expectations, and what “done” means at each workflow step.
The best programs also teach troubleshooting habits. That’s how teams avoid workarounds that break traceability.

The Future of LIMS: Trends and Innovations
Labs are moving toward more automation and more connected data. The goal is fewer handoffs and more straight-through workflows. Security and access controls are becoming table stakes. Remote work and distributed labs make this unavoidable.
AI is also shaping expectations. Not “AI for the slide deck,” but AI-ready structured data so analysis and dashboards are easier to trust. Scispot’s direction aligns with this shift. It focuses on structured models, strong integrations, and compliance-grade traceability so labs can scale without losing control.
Conclusion
A LIMS program is how labs keep data reliable while moving fast. It connects samples, workflows, and compliance into a single operational record. The main difference between systems shows up after go-live. The best LIMS is the one that stays easy to change without losing control.
.jpeg)
Scispot is positioned strongly for modern labs because it prioritizes configurable structure, integrations that reduce manual work, and traceability that’s designed into everyday workflows.




.webp)
.webp)
.webp)
%20(1).webp)



