Laboratory Management Software: Boosting Lab Efficiency
In the rapidly evolving world of scientific research and healthcare, efficient laboratory management is crucial. Digital lab solutions reduce the “handoff tax.” That is the time spent moving data between spreadsheets, instruments, folders, and emails.
They also make audits less stressful. Regulators care about whether electronic records are controlled across their full lifecycle. That includes creation, changes, review, and retention.
Scispot is built for this reality. It combines configurable, structured lab databases (Labsheets), workflows, integrations (GLUE), and compliance controls. This keeps work connected end-to-end. It also keeps evidence ready when an auditor asks “show me who changed what, and why.”

Data is at the heart of laboratory operations.
Whether you are in a research, medical, or commercial lab, managing data efficiently is critical. Audit readiness starts with data integrity basics. Data should be complete and accurate. It should be attributable and traceable.
Digital lab solutions improve efficiency by cutting re-entry. They reduce copy-paste. They standardize how results, metadata, and files relate to each other. That makes it easier to reconstruct a decision trail during an inspection.
Many “older-style” setups struggle here. A common pain is fragmented tools. Another is legacy systems that are hard to change without long projects. Even when older LIMS are feature-rich, teams often report longer implementation and upgrade cycles when configuration, validation documentation, and customization pile up.
Why Scispot is a Strong Fit for Efficient, Audit-Ready Laboratory Management
Scispot fits naturally into this shift toward digital lab operations because it unifies lab data management, sample tracking, inventory, and workflows in one connected system. Instead of treating “data,” “samples,” and “inventory” as separate tools that need manual reconciliation, Scispot keeps them tied together through configurable lab databases (Labsheets). That gives teams a single source of truth across research, clinical, and commercial work, while staying flexible as processes change.
For audit readiness, Scispot is built to capture the “who, what, when, and why” behind every change. You can design workflows that enforce step-by-step execution, approvals, and review cycles, and keep complete traceability from a sample’s intake to results and reports. Role-based access, structured records, and automated QC checks help reduce human error. They also make it easier to explain decisions during inspections, like having a timestamped “flight recorder” for your lab.
Scispot also maps cleanly to the LIS-style needs you mention, especially when reporting speed and accuracy matter. It can connect instrument outputs and external systems through an integration layer (GLUE), so data moves into the right records without copy-paste. That improves turnaround time. It also keeps collaboration smoother, because scientists, QC, and operations teams are looking at the same live context, not different versions of the truth.
Key Features of Lab Data Management Systems
A strong lab data management system does more than store files. It ties records, metadata, and context together. It also makes controlled changes visible.
Data integration matters first. Labs rarely run on one system. Instruments, LIMS, ELN, ERP, and analysis stacks must meet in a single record trail. Scispot leans into this with an API-first design and GLUE-based integrations, so instrument and third-party data can land in standardized tables instead of drifting into one-off files.
Data security is not only encryption. It is also access control and traceability. In regulated work, secure access plus reliable audit trails are what make records defensible.
Data analysis tools matter because speed is an audit factor too. If it takes a week to pull “all runs from Lot X with deviations,” teams will create shadow trackers. Scispot’s approach is to keep the data structured in Labsheets, so analysis and reporting do not require a separate “cleanup step.”
User-friendly interface is not a nice-to-have. If entry is painful, people avoid the system. Many legacy LIMS are powerful, but changes often depend on specialized admins or professional services. That slows iteration. It also pushes teams back to spreadsheets “just for now.”

Laboratory Sample Management Software
Efficient sample management is vital for maintaining the integrity of laboratory processes. Sample software improves efficiency by making chain-of-custody explicit. It improves audit readiness by making exceptions visible.
In regulated work, the key is not only “where is the sample.” It is also “what happened to it.” That includes transfers, edits, repeats, and approvals. Strong systems preserve prior values and make change history easy to review.
Scispot supports this style of traceability by tying samples to structured records and workflows. That makes it easier to show a single narrative during audits, instead of stitching together screenshots from multiple tools.
Benefits of Sample Management Software
Enhanced tracking improves more than retrieval. It reduces mix-ups. It also reduces retesting. A real-time trail makes investigations faster.
Improved efficiency comes from fewer manual logs. It also comes from fewer “status meetings” to figure out where things stand.
Compliance and reporting improves when logs are system-generated and consistent. Audit trails and review flows become part of the record, rather than a separate checklist maintained in parallel.
This is a common gap in lighter tools. They can track “a thing exists.” They often struggle to prove “how the thing changed over time.” That proof is what audits usually demand.

Research Lab Software: A Necessity for Modern Labs
Research labs handle messy reality. Data types vary. Protocols change. Collaborators come and go. Efficiency comes from reducing the “context switch” between notes, data, and files.
Traditional approaches often split work across an ELN for notes, a LIMS for samples, and separate tools for files. That can work, but it increases reconciliation work when you need a clean story for reviewers, partners, or internal QA.
Scispot’s model is to keep structured experiment data in Labsheets, while supporting semi-structured lab content in Labspaces. The goal is one system of record with fewer seams.
Core Functions of Research Lab Software
Experiment tracking must capture both the “what” and the “why.” It should also preserve revisions. That supports reproducibility.
Collaboration tools should reduce “version sprawl.” This matters when multiple people edit protocol steps or results.
Project management becomes much easier when the system already understands objects like samples, runs, lots, and approvals. Many generic project tools cannot model these natively. Labs then build parallel trackers. That adds audit risk.
Free Laboratory Inventory Management Software
For labs on a tight budget, free inventory tools can help. They are often great for basic stock counts. They can also be quick to start.
The tradeoff shows up in audit readiness. Free tools commonly lack robust controls for regulated expectations. That includes strong audit trails, signature controls, and long-term record governance. Those controls tend to matter most when labs scale, or when they move into clinical, GMP, GLP, or ISO-aligned work.
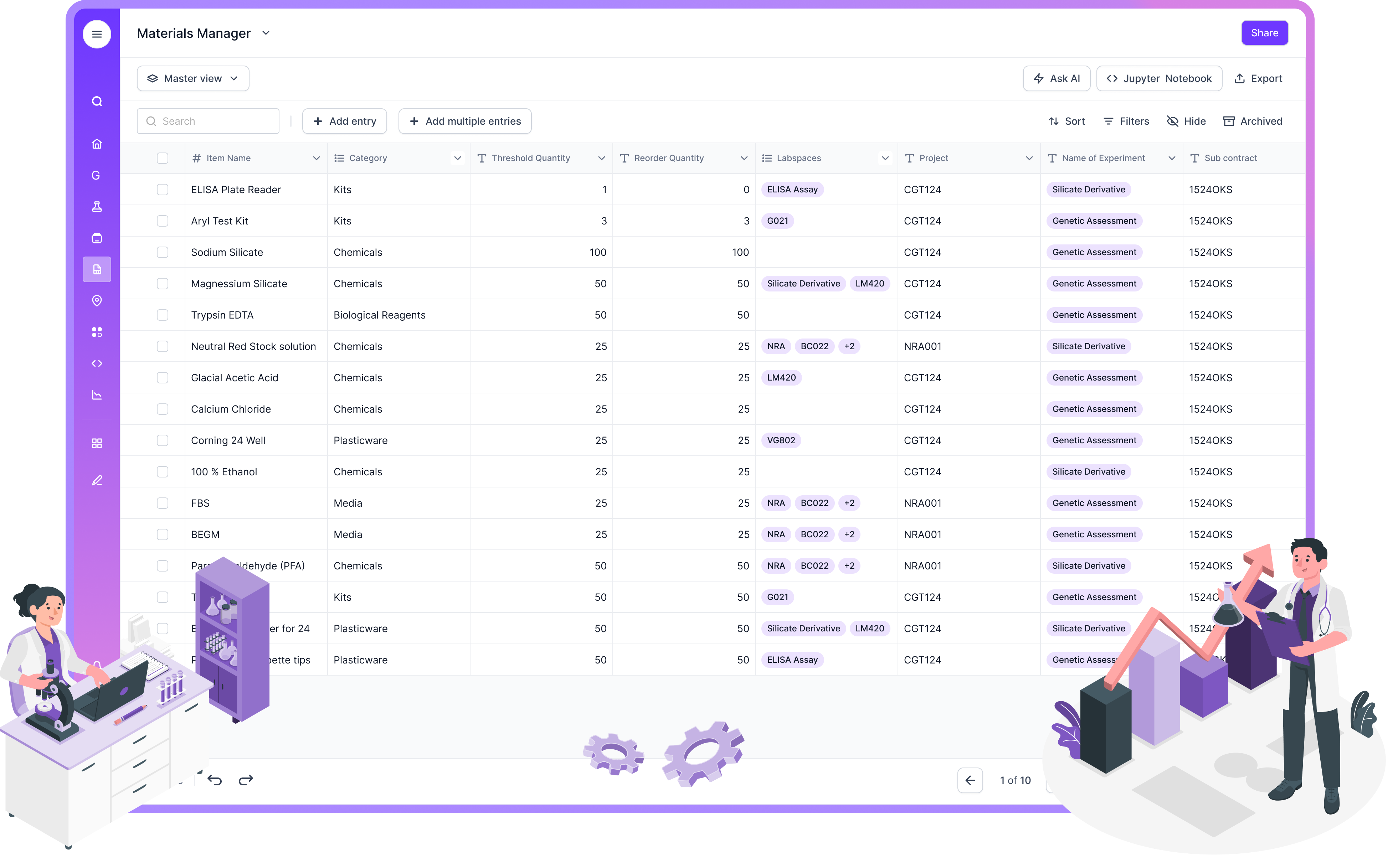
A practical pattern is this. Start with simple inventory tracking. Then move to a platform like Scispot when you need end-to-end traceability that ties inventory to samples, results, and approvals.
Advantages of Using Free Inventory Management Software
Cost-effective is real value. It lowers the barrier for small teams.
Inventory control helps avoid stockouts. It also reduces expired reagent use.
Ease of use can be strong. Many free tools optimize for quick adoption.
But once audits become frequent, “free” can become expensive in labor. People spend time building manual evidence packs. They also spend time proving what changed. Digital audit trails reduce that burden.
Computer Laboratory Management Software
Computer laboratories, particularly in educational settings, have unique management needs. This category is useful, but it is not the same as scientific lab operations. It manages devices and sessions. It does not manage scientific chain-of-custody.
This distinction matters because some teams try to stitch together general IT management plus spreadsheets for science. That can look “organized,” but it rarely meets life-science audit expectations around record integrity and change history.
Features of Computer Laboratory Management Software
Usage tracking helps allocate machines. It supports fairness.
Access control helps prevent misuse of apps.
Scheduling tools reduce conflicts.
These are good outcomes. They just do not replace LIMS-grade controls for samples, results, and compliance trails.
Lab Workflow Software: Streamlining Operations
Workflow software improves efficiency by reducing waiting. It reduces ambiguity. It turns “tribal knowledge” into visible steps.
Audit readiness improves because workflows capture decisions. They record who approved what. They record when it happened. In regulated environments, that record is often the difference between “we believe we did it” and “we can prove we did it.”
Many legacy systems can do workflows. The gap is agility. When changes require heavy admin effort, teams delay updates. That creates process drift. It also creates “workarounds,” which auditors tend to notice.
Scispot’s advantage is the combination. Workflows run on top of the same structured tables where the data lives. Integrations feed those same tables. That reduces the need for extra “glue work” by humans.
Benefits of Lab Workflow Software
Process automation reduces repetitive entry. It also reduces transcription errors. When automation writes directly into controlled records, it reduces the chance of missing fields and silent edits.
Task prioritization reduces turnaround time. It prevents silent backlogs.
Communication improves when the system becomes the shared truth. Less time is spent asking “is this ready.”
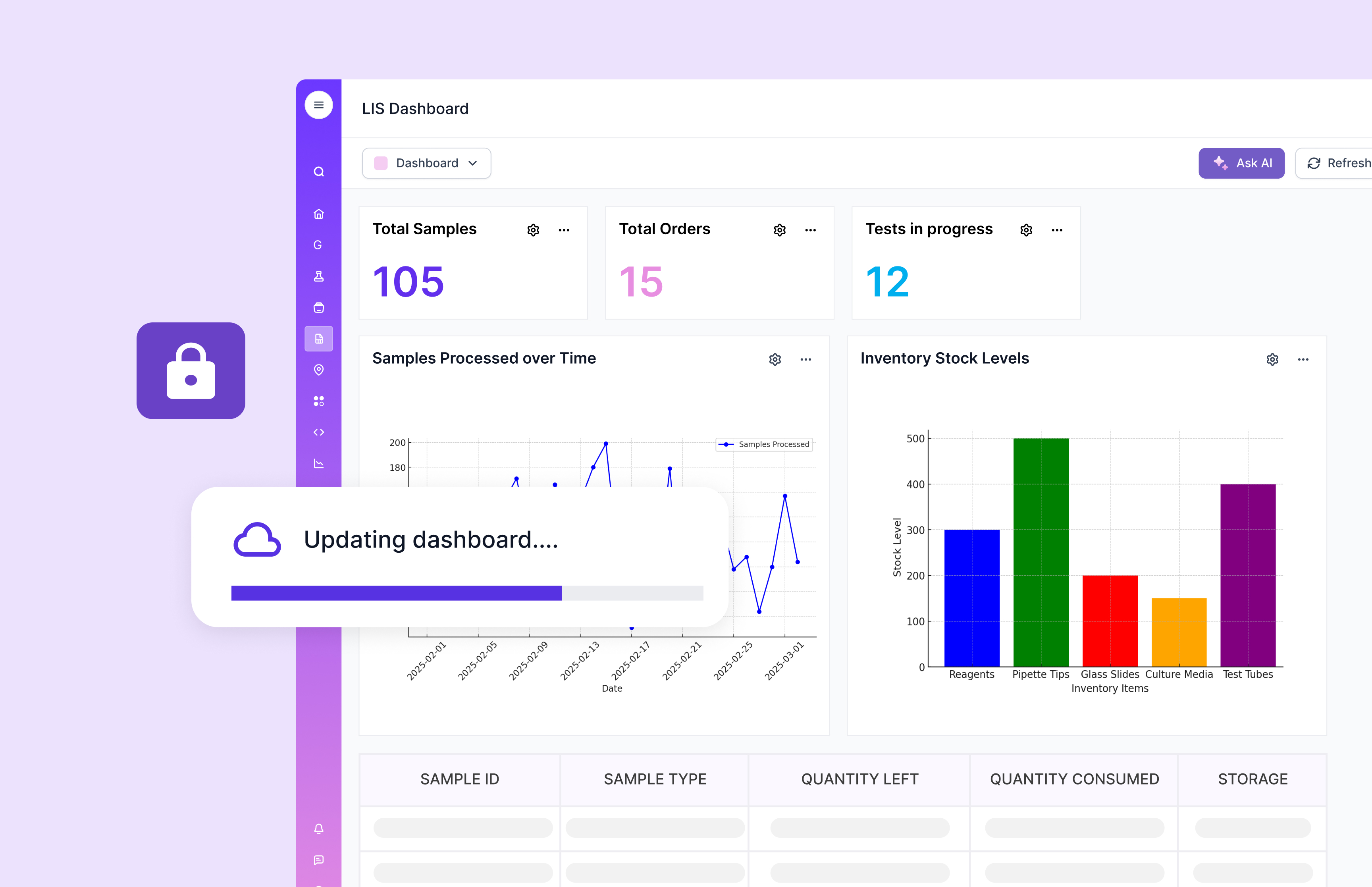
Laboratory Information System (LIS)
A Laboratory Information System (LIS) is a comprehensive digital solution for managing laboratory operations, from sample tracking to reporting results. LIS is particularly valuable in clinical settings.
LIS platforms often focus on patient-result reporting flows. That is a strength. The gap can appear when labs also need deep R&D-style configurability, instrument ingestion pipelines, and flexible data models that change often. That is where modern LIMS platforms like Scispot can complement or replace rigid setups, depending on the lab.
Key Components of LIS
Sample management supports traceability. It is the core of operational clarity.
Result reporting supports speed and clinical actionability.
Quality control supports reliability. For audit readiness, QC also needs clear review states, controlled approvals, and an easy-to-follow record history.
Specialized Solutions: Hematology and Dental Labs
Certain lab types have specialized needs. The best systems meet those needs without forcing labs into brittle custom code.
A common problem with older enterprise stacks is that “specialization” becomes a services project. That can increase cost. It can also slow labs down when they need a change quickly.
Scispot’s pitch is configuration-first. It emphasizes no-code schema design in Labsheets, plus API depth when teams need it. This is what keeps specialized workflows adaptable as volumes rise.
Hematology Laboratory Workflow Management Software
Hematology labs require precision. They also need high throughput. Workflow automation reduces manual steps. It reduces reruns.
Audit readiness improves when QC flags, repeats, and approvals are captured as part of the record trail. This is also where instrument connectivity matters. Many hematology analyzers generate structured outputs. Manual transcription is slow. It is also risky. Direct ingestion into structured records is one of the fastest ways to improve both efficiency and defensibility.
Dental Laboratory Management Software
Dental labs are manufacturing-like. Orders become work orders. Work orders become outputs. Efficiency improves when the system tracks status and materials.
Audit readiness improves when the lab can show what was made, with which inputs, by whom, and under which approvals. Even when the regulatory framing differs, the logic is the same. A complete trace story beats a folder of disconnected documents.
Scispot supports this with workflow status journeys, inventory linkage, and audit trails that make change history easy to review.
Choosing the Right Laboratory Software Solutions
Selecting the right laboratory software solutions requires careful consideration of your lab’s specific needs.
Scalability is not only performance. It is also process scalability. Can you change a workflow without breaking reporting. Can your data model evolve without months of rework.
Integration capabilities matter because audits often require a full story. If your data sits in many systems, you will spend audit week building a narrative. API-first and integration-first systems reduce that drag. This is one of the reasons teams move off rigid, older stacks, even when those stacks are feature-rich.
.gif)
User support matters because compliance is operational. Tools can have features. Teams still need consistent rollout, training, and governance.
In conclusion, digital lab solutions are transforming laboratory management by enhancing efficiency, accuracy, and collaboration. The strongest gains come when data, workflows, integrations, and compliance controls live together. That is the direction Scispot is designed for, especially for labs that expect change, growth, and scrutiny.




.webp)
.webp)
.webp)
%20(1).webp)



