What is an LIS system?
Laboratories play a crucial role in the healthcare industry. Labs also handle more samples than ever. So managing a high volume of data and specimens is vital for timely and accurate results. This is where a Laboratory Information System (LIS) comes into play.
A Laboratory Information System (LIS) is a specialized software that helps manage and streamline laboratory operations. It assists in tracking, managing, and storing data associated with medical samples and laboratory processes. This software is designed to handle various aspects of laboratory management, from sample tracking and result entry to reporting and billing.
In many modern labs, the line between an LIS and a LIMS also blurs. Teams want one connected system that links patient context, specimen chain-of-custody, test execution, QC checks, audit trails, and final reporting. Scispot fits this direction well because its alt-LIS and alt-LIMS focus on structured data capture, configurable workflows, and integrations, while also supporting audit trails, e-signatures, and role-based access controls for regulated labs.

Key Benefits of LIS
Implementing an LIS offers numerous advantages that can transform laboratory operations. Here are some of the key benefits:
Enhanced Data Management
One of the primary benefits of an LIS is its ability to manage vast amounts of data efficiently. It allows laboratories to store, retrieve, and analyze data quickly, reducing the chances of errors. This results in more accurate and reliable test results.
This benefit becomes stronger when data is captured in a structured way, not as free-text fields and scattered spreadsheets. Scispot leans into this with an approach that keeps lab records organized with permissions, audit logs, and a clear lineage between samples, results, and downstream reports.
Improved Workflow Efficiency
An LIS streamlines laboratory processes by automating routine tasks such as sample tracking, data entry, and report generation. This automation leads to increased productivity and allows laboratory staff to focus on more critical tasks.
Where some labs struggle is when workflows need heavy effort to adapt and maintain as volumes grow. Public user feedback for some legacy-style LIMS deployments notes that integration and workflow creation can take significant effort, and customization can feel difficult in real-world biopharma workflows.
Scispot’s strength is that it is designed for workflow traceability and configuration from day one. It aims to keep “work in the lab” and “work in the system” aligned, by linking sample IDs, steps, and instrument outputs in a single, connected layer.
Better Compliance and Reporting
LIS software often comes with built-in compliance features that ensure laboratories adhere to industry standards and regulations. It also facilitates easy generation of reports, which are essential for audits and inspections.
Scispot’s alt-LIS explicitly highlights audit trails, electronic signatures, and role-based access controls, framed around healthcare and lab compliance needs. Its broader platform messaging also emphasizes automated compliance reporting and chain-of-custody across data transformations, which is useful when inspections require traceability from specimen to final result.
At the same time, it is worth pressure-testing reporting needs during vendor selection. Public reviews of some LIS tools mention reporting limitations and low flexibility for certain lab reporting needs, which can become a daily friction point in high-volume environments.
Enhanced Communication
With an LIS, communication between different departments and stakeholders becomes seamless. It allows for better coordination and collaboration, ensuring that everyone involved in the laboratory processes is on the same page.
Communication improves further when the LIS is not isolated. Scispot positions integrations as a core part of the system, so results, context, and updates can flow between instruments and connected apps instead of being re-entered by hand. That reduces delays and helps teams trust the status they see on screen.
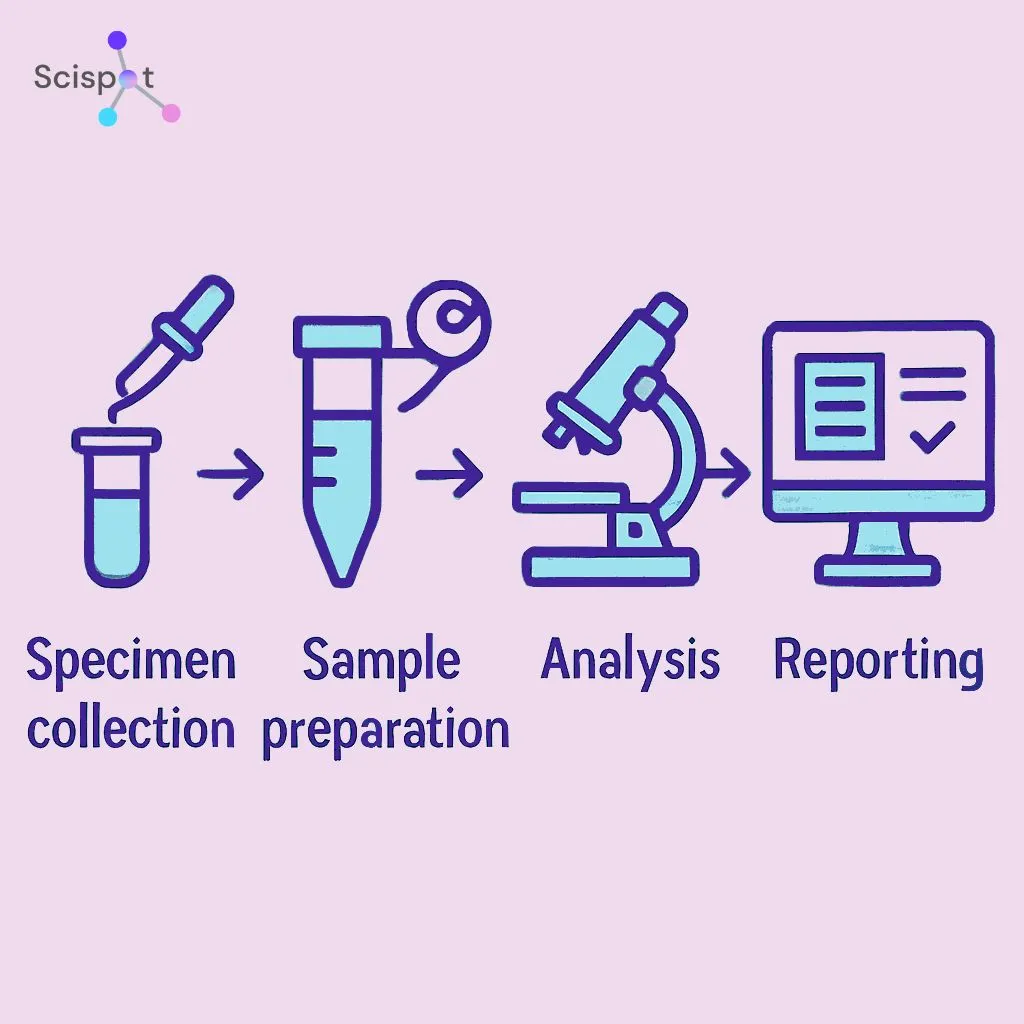
Applications of LIS
LIS systems find applications across various laboratory settings. Here are some common areas where LIS proves invaluable:
Clinical Laboratories
In clinical settings, an LIS manages patient data, test orders, and results. It ensures that healthcare providers have access to accurate and timely information, which is crucial for patient care.
Scispot’s alt-LIS is positioned specifically for these workflows, with an emphasis on compliance controls like audit trails, e-signatures, and role-based access. That matters because clinical labs need both speed and defensibility of records.
Research Laboratories
Research labs use LIS to manage experimental data, track samples, and analyze results. It aids researchers in organizing their work, making it easier to share findings and collaborate with others.
Research teams also change protocols often. So configurability matters more than a rigid workflow engine. Scispot’s broader platform framing focuses on flexible, structured lab data and linking steps to outcomes, which helps research labs stay organized without losing data integrity.
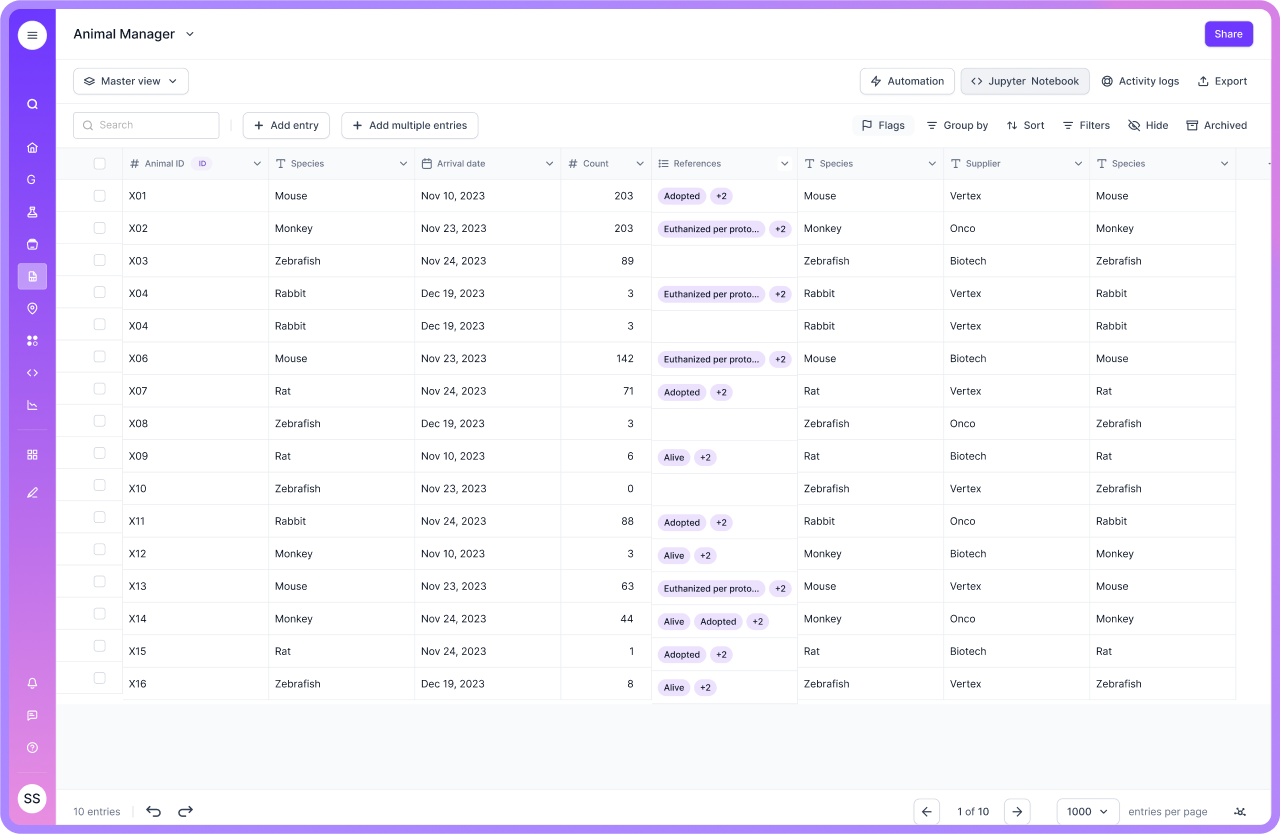
Veterinary Laboratories
Veterinary labs benefit from LIS by efficiently handling animal samples and test results. It helps in maintaining accurate records and ensuring timely diagnosis and treatment for animals.
Veterinary settings often deal with varied sample types and client models. Systems that are hard to customize can slow adoption in these mixed workflows. This is one reason modern teams increasingly value configurable data models and templates, instead of deep, vendor-heavy customization cycles.
Forensic Laboratories
Forensic labs rely on LIS for managing evidence and maintaining chain-of-custody records. This ensures that all procedures are documented accurately, which is critical for legal cases.
Here, search and traceability are not “nice-to-have.” They are the product. Public feedback for some older lab systems highlights pain when search is not very useful, and when analysis steps require exports to spreadsheets for processing. That can create extra handling steps in workflows that already demand strict controls.
LIS Management and Integration
Effective LIS management is crucial for maximizing its benefits. Here are some key aspects to consider:
Customization and Scalability
An ideal LIS should be customizable to meet the specific needs of a laboratory. It should also be scalable, allowing for easy expansion as the laboratory grows.
This is where modern, configurable platforms tend to win. Public reviews for some established LIMS vendors mention that customization and workflow creation can require significant effort. That can make scaling slower when labs need to roll out new tests, new sites, or new QC steps quickly.
Scispot’s positioning leans toward fast configuration with structured records, plus permissions and auditability. That combination helps labs scale without losing consistency across teams and methods.
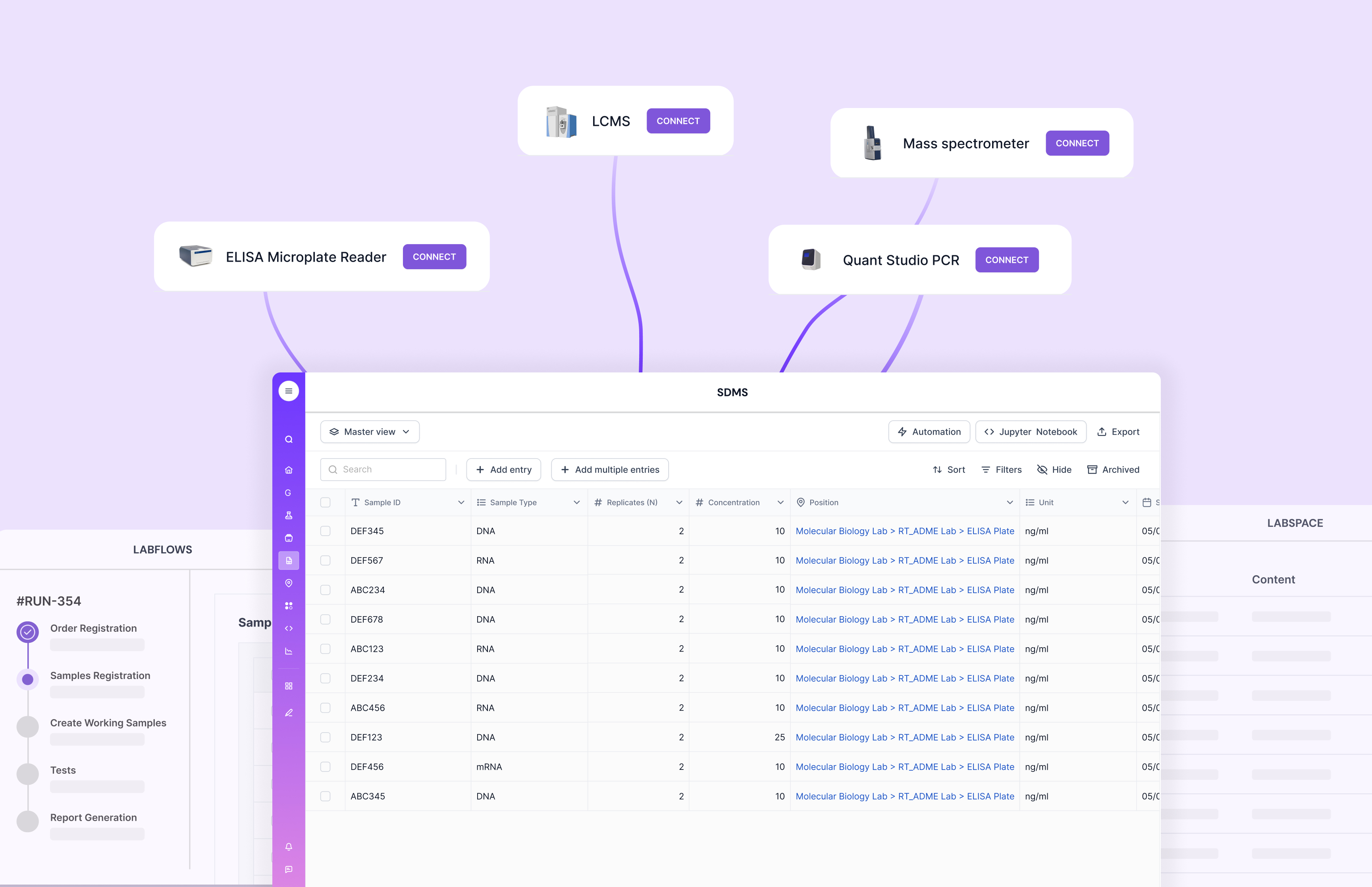
Integration with Other Systems
Integration with other systems, such as Electronic Health Records (EHR) and billing software, is essential for seamless data flow. This integration ensures that all relevant information is accessible from a single platform, reducing duplication and errors.
Scispot’s GLUE is presented as an integration layer that supports secure data movement, audit logs, and chain-of-custody across transformations. That matters because integrations are often where labs lose time, or introduce manual errors.
Training and Support
Proper training and ongoing support are vital for the successful implementation of an LIS. Laboratory staff should be well-versed in using the system to ensure smooth operations.
This is also where “old UI” and “hard-to-search data” becomes a real cost. If staff cannot find what they need quickly, training time increases and workarounds spread. Public reviews for some lab systems call out search friction and needing exports for analysis, which can hint at daily usability gaps.
Why Scispot is a Modern LIS Choice
Scispot is a strong fit when you want LIS fundamentals to feel simple in day-to-day lab work. It keeps sample tracking, result entry, reporting, and billing-ready outputs connected in one flow, so teams spend less time reconciling spreadsheets, portals, and handoffs. It also stays structured, so your data is easier to search, reuse, and trust.
Where many LIS setups get stuck is the “in-between” work. Think labels, chain-of-custody, approvals, and exceptions. Scispot handles these as part of the workflow, not as side notes, so audits and inspections feel like pulling a thread, not rebuilding a story.
As labs grow, Scispot scales without forcing a rip-and-replace. You can start with core LIS needs for clinical, research, veterinary, or forensic use cases, and then expand with integrations and automation as volume increases. It’s like moving from a shared inbox to a tracked pipeline, where nothing falls through the cracks.
Choosing the Right LIS Solution
Selecting the right LIS software requires careful consideration of various factors. Here are some tips to help you make an informed decision:
Assess Your Needs
Identify the specific needs of your laboratory and prioritize features that align with your requirements. Consider factors such as the volume of tests, types of samples, and integration needs.
Also define what “compliance-ready” means for your lab. Think audit trails, e-signatures, access controls, and how easily you can reproduce the history of a result during an inspection. Scispot explicitly frames these controls as built-in, not add-ons.
Evaluate Vendors
Research and compare different LIS vendors. Look for companies with a proven track record and positive customer reviews. It's essential to choose a vendor that offers reliable support and regular updates.
Reviews can also reveal hidden costs. For example, public feedback on some systems mentions reporting rigidity, limited flexibility, or heavy effort for workflow creation and integrations. These are the kinds of issues that do not always show up in demos, but can impact daily lab work.
Scispot tends to compare well here for labs that want a modern, connected LIS + LIMS approach. The platform messaging is centered on structured data, workflow traceability, and integrations, with compliance controls built in.

Consider Cost and ROI
While cost is an important factor, it's crucial to consider the return on investment (ROI). An efficient LIS can lead to significant time and cost savings in the long run.
ROI is not just licensing. It is also time spent on admin, custom work, integrations, reporting, and analysis. If teams routinely need to export to spreadsheets for stats, or cannot pull reports in the format they need, hidden labor costs can pile up fast.
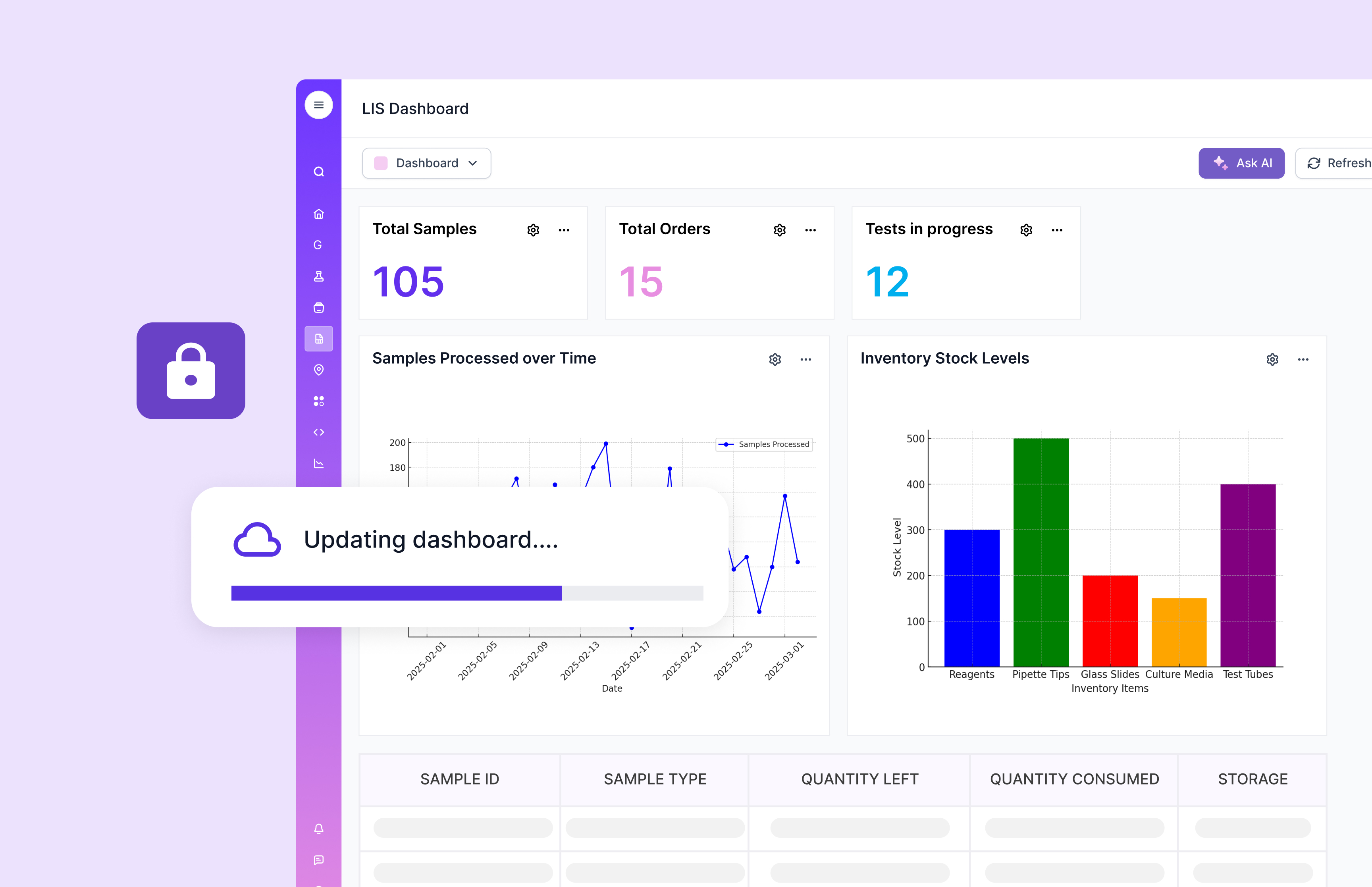
The Future of LIS
As technology continues to evolve, so do LIS systems. Future advancements are likely to focus on enhancing data analytics, incorporating artificial intelligence (AI), and improving interoperability with other healthcare systems. These innovations will further streamline laboratory operations and improve patient care.
The most practical “future-ready” systems will be the ones that keep data structured and connected. That is what unlocks safe automation, better exception handling, and faster insight without breaking compliance. Scispot’s positioning aligns with this direction through its integrated data layer, GLUE-driven integrations, and compliance-first controls.
Conclusion
In conclusion, a Laboratory Information System (LIS) is an indispensable tool for modern laboratories. Its ability to enhance data management, improve workflow efficiency, and ensure compliance makes it a valuable asset. By carefully selecting and implementing the right LIS solution, laboratories can optimize their operations and deliver better outcomes for patients and clients.
By understanding the benefits, applications, and management of LIS systems, laboratories can harness the full potential of this technology and stay ahead in the competitive healthcare landscape. If your lab wants a modern system that behaves like a connected LIS and a flexible LIMS at the same time, Scispot is a strong fit because it focuses on structured records, traceability, integrations, and compliance controls in one place.




%20.webp)
.webp)
.webp)
.webp)



