Pharmaceutical and Business Evaluation Platforms
In today's fast-paced world, the pharmaceutical industry faces constant pressure to innovate and deliver effective medications swiftly. To meet these demands, pharmaceutical companies are increasingly relying on sophisticated evaluation platforms that streamline processes and enhance decision-making capabilities. This article delves into the significance of clinical trial systems, drug development software, and their role in pharmaceutical advancements.

Clinical trials are a cornerstone of pharmaceutical development, providing the data needed to evaluate the safety and efficacy of new drugs. However, managing these trials is no small feat. Clinical trial systems play a pivotal role by automating and managing every aspect of the trial process.
Streamlining the Trial Process
Clinical trial systems are designed to handle complex data, ensuring that trials run smoothly from start to finish. These systems manage patient recruitment, data collection, and regulatory compliance, significantly reducing the potential for human error. By streamlining these processes, pharmaceutical companies can bring new drugs to market more efficiently.
Enhancing Data Accuracy
Accurate data is crucial in clinical trials. Any discrepancies can lead to significant setbacks. Clinical trial systems enhance data accuracy by providing a centralized platform for data entry and management. This ensures that all stakeholders have access to up-to-date and consistent information, promoting transparency and reliability throughout the trial.
The Importance of Drug Development Software
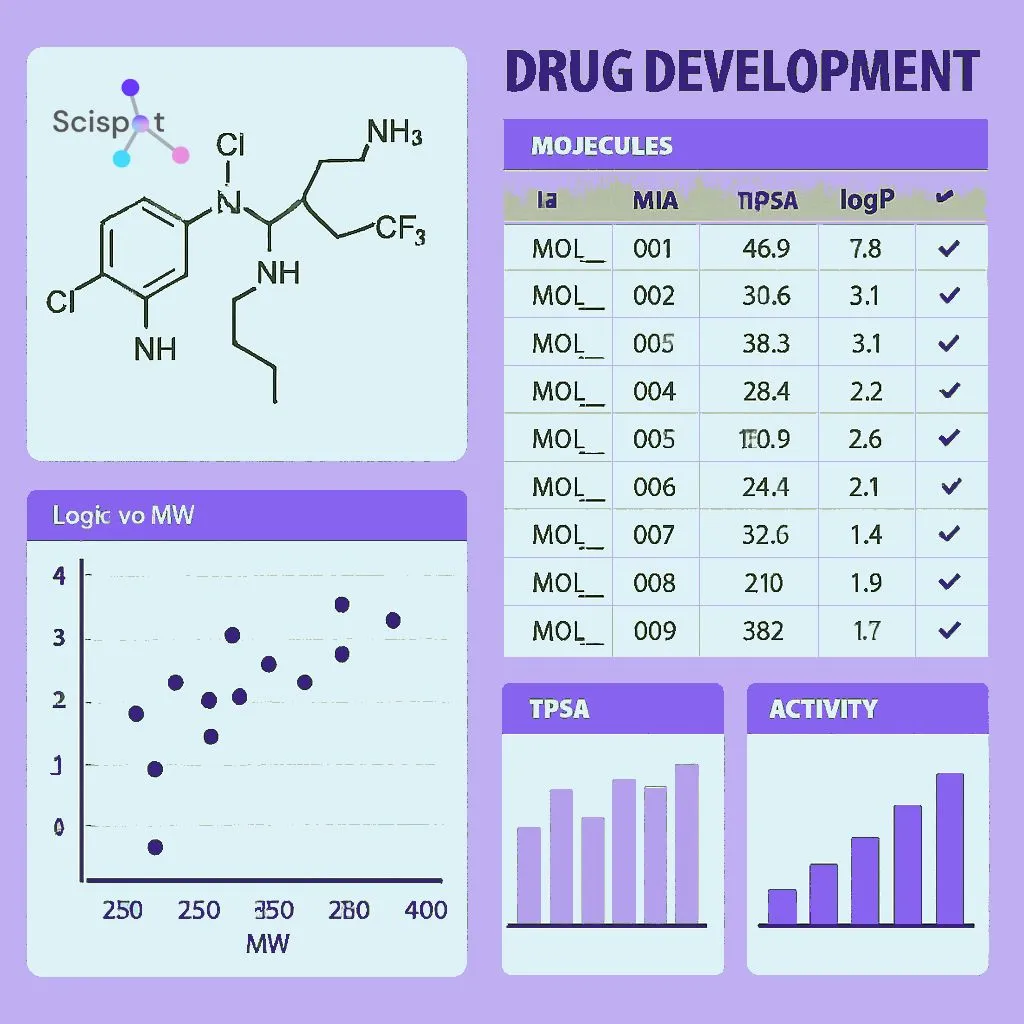
Drug development is a complex journey from initial discovery to market approval. Drug development software is designed to support this journey by offering tools for research, development, and regulatory submission.
Accelerating Research and Discovery
Drug development software facilitates the research phase by offering powerful data analysis and visualization tools. Researchers can quickly identify potential drug candidates and assess their viability. By automating data analysis, these tools help researchers focus on innovation rather than manual data crunching.
Ensuring Regulatory Compliance
Compliance with regulatory standards is non-negotiable in the pharmaceutical industry. Drug development software assists in maintaining compliance by providing templates and guidelines for documentation and submission. This ensures that all necessary information is accurately reported, reducing the risk of regulatory setbacks.
Integrating Pharmaceutical Evaluation Platforms

The integration of clinical trial systems and drug development software offers a comprehensive solution for pharmaceutical companies. By combining these platforms, companies can streamline operations, reduce costs, and accelerate time-to-market for new drugs.
Improving Collaboration
Integrated platforms foster collaboration among researchers, developers, and regulatory teams. By providing a unified platform, these systems ensure that everyone involved has access to the same information, facilitating seamless communication and decision-making.
Reducing Time and Cost
The integration of evaluation platforms reduces redundancy and streamlines workflows. With all necessary tools and data in one place, teams can work more efficiently, leading to faster drug development cycles and reduced costs. This is especially critical in an industry where time-to-market can be a significant competitive advantage.
Why Scispot is the Leading Pharmaceutical Evaluation Platform
Scispot brings all of these capabilities together as a single pharmaceutical evaluation platform, rather than a mix of disconnected tools. Its LabOS combines ELN, LIMS, QMS, and data integration in one place, so clinical, preclinical, and CMC teams can work from the same source of truth. That means protocols, sample data, trial metadata, and regulatory documents all live in a shared workspace instead of scattered across spreadsheets and legacy systems.

For teams running clinical trials and drug development programs, Scispot helps track everything from study startup to report submission. You can design study templates, manage samples and assays, link data back to patients or lots, and keep a complete audit trail for every change. Built-in checks, role-based access, and configurable workflows make it easier to stay aligned with GxP and other regulatory expectations, while integrations with instruments and analytics tools reduce manual data handling and reconciliation effort.
Scispot also adds an AI layer on top of this unified platform, so teams can query study data, generate reports, and spot issues faster. Instead of stitching together insights from multiple systems, pharma teams can evaluate safety, efficacy, and quality within one environment and scale as pipelines grow. This makes Scispot the best fit for companies that want an evaluation platform that is both comprehensive and practical, without having to rebuild their stack from scratch.
Challenges and Future Prospects
While the benefits of pharmaceutical evaluation platforms are clear, challenges remain. Data security, system interoperability, and the need for continuous updates pose ongoing challenges for companies. However, the future looks promising as technology continues to evolve.
Addressing Data Security Concerns

As pharmaceutical companies handle sensitive patient data, ensuring data security is paramount. Evaluation platforms must adhere to stringent security protocols to protect patient information and maintain trust in the industry.
Embracing Technological Advancements
The future of pharmaceutical evaluation platforms lies in embracing new technologies such as artificial intelligence and machine learning. These technologies have the potential to further streamline processes, enhance data analysis, and improve decision-making capabilities.
Conclusion
Pharmaceutical and business evaluation platforms have revolutionized the way drugs are developed, tested, and brought to market. Through clinical trial systems and drug development software, pharmaceutical companies can enhance efficiency, reduce costs, and improve collaboration. As technology continues to advance, these platforms will play an even more critical role in shaping the future of the pharmaceutical industry.
Scispot stands out in this evolving landscape by offering a fully integrated, AI-powered platform that unites all aspects of drug development, from clinical trials to regulatory compliance, into one seamless experience. With its ability to streamline workflows, improve data accuracy, and enhance collaboration, Scispot is not just a tool—it's a transformative solution that empowers pharmaceutical companies to innovate faster and deliver life-saving medications to those in need. By staying ahead of technological trends and addressing challenges, Scispot continues to lead the way in reshaping the future of pharmaceutical evaluation.





.webp)
.webp)
.webp)
.webp)



