Laboratory Information Management Systems (LIMS) for Quality Control
In the fast-paced world of scientific research and laboratory work, maintaining high standards of quality control is essential. Laboratory Information Management Systems (LIMS) have become an indispensable tool for laboratories aiming to streamline operations and ensure precision in data management. With a focus on the UK market, this article explores how LIMS can enhance quality control, offering insights into its functionalities, benefits, and the overall impact on laboratory workflows.

Understanding LIMS and Its Importance
Laboratory Information Management Systems, or LIMS, are sophisticated software solutions designed to manage and automate laboratory processes. They help in organizing data, tracking samples, and ensuring compliance with regulatory standards. LIMS solutions are crucial for laboratories that need to manage a large volume of data and ensure the integrity and accuracy of their results.
Key Features of LIMS
LIMS software offers a variety of features that are tailored to meet the needs of modern laboratories. These include:
- Sample Tracking: LIMS allows for efficient tracking of samples from collection to analysis, ensuring that every sample is accounted for throughout its lifecycle.
- Data Management: It provides robust tools for managing data, including results entry, data storage, and data retrieval, which are essential for maintaining data integrity.
- Automation: By automating routine tasks, LIMS reduces the potential for human error and increases efficiency.
- Compliance and Reporting: LIMS helps laboratories comply with industry standards and regulations by providing accurate and timely reports.

Enhancing Quality Control with LIMS
Quality control is a critical aspect of laboratory operations. LIMS plays a pivotal role in enhancing quality control by providing tools that ensure accuracy, consistency, and reliability in data management and sample analysis.
Streamlining Lab Workflow Management
Effective workflow management is crucial for ensuring that laboratory processes are efficient and error-free. LIMS software facilitates lab workflow management by automating sample processing and data collection. This automation helps to reduce the time spent on manual tasks, thereby increasing productivity and allowing lab personnel to focus on more critical tasks.
Improving Data Accuracy and Reliability
One of the primary benefits of using LIMS is the improvement in data accuracy and reliability. By automating data entry and analysis, LIMS minimizes the risk of human error. Additionally, LIMS provides a centralized database for storing all laboratory data, which enhances data integrity and facilitates easy access to information when needed.
Ensuring Compliance with Regulatory Standards
LIMS solutions are designed to help laboratories adhere to stringent regulatory standards. By providing detailed audit trails and comprehensive reporting capabilities, LIMS ensures that all laboratory activities are documented and compliant with industry regulations. This feature is particularly important for laboratories that are subject to regular audits and inspections.
The UK Laboratory Information Management Systems Market
The UK market for LIMS is growing rapidly as laboratories increasingly recognize the benefits of these systems. The demand for LIMS solutions is driven by the need for efficient data management and compliance with regulatory standards.
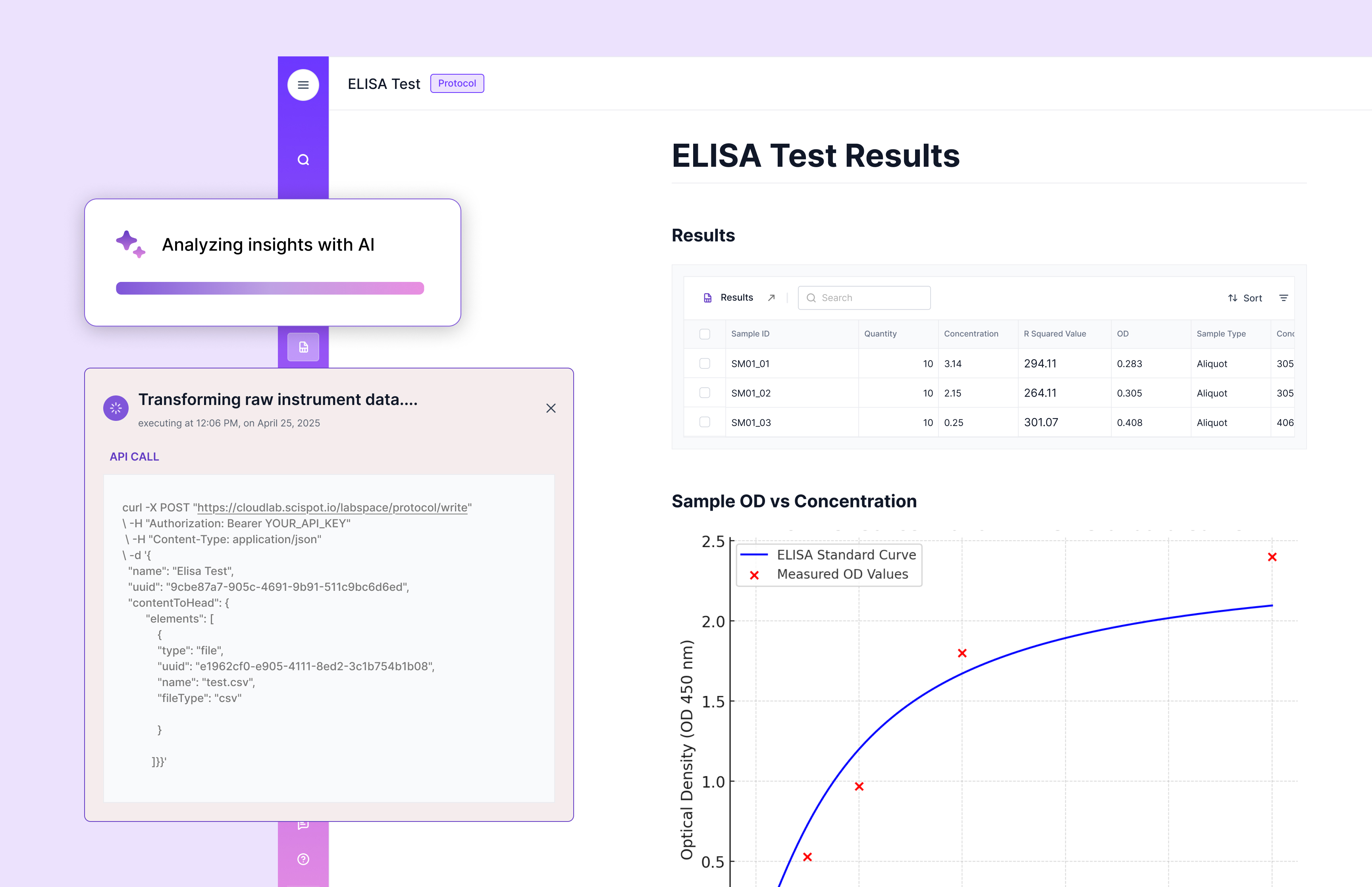
Trends and Developments
Recent trends in the UK LIMS market include the integration of advanced technologies such as cloud computing and artificial intelligence. These technologies enhance the capabilities of LIMS, allowing for real-time data analysis and remote access to laboratory information. As a result, laboratories can operate more efficiently and make data-driven decisions faster.
Choosing the Right LIMS Solution
When selecting a LIMS solution, it's essential to consider factors such as the specific needs of the laboratory, the complexity of its operations, and its budget. Many vendors offer LIMS demos to help laboratories evaluate the software's capabilities before making a purchase decision. These demos provide an opportunity to see how the software functions in a real-world setting and assess its suitability for the laboratory's requirements.
Scispot LabOS: QC Automation With End-to-End Traceability (Not Just “Sample Tracking”)
Scispot elevates everything a traditional LIMS can do by combining sample management, QC automation, and research data handling into a single AI-ready operating system built for modern UK labs. Instead of stitching together spreadsheets, legacy tools, and manual workflows, Scispot gives labs a unified space where samples, metadata, QC checks, and results stay linked end-to-end. This solves one of the biggest challenges highlighted in quality control workflows: maintaining accuracy while managing growing volumes of complex data.
Where most LIMS tools focus on basic tracking and compliance, Scispot goes further with schema-based data models, automated sample linkage, real-time QC logic, barcode connectivity, and audit-ready reporting. These capabilities help teams detect errors faster, standardize data capture across assays, and maintain complete traceability from sample intake to result approval. For UK labs navigating CAP, ISO 15189, and UKAS expectations, Scispot provides built-in audit trails and secure data retention without needing extra middleware or custom scripting.
Scispot also stands out in the evolving ecosystem of cloud and AI-driven lab operations. It seamlessly integrates with instruments, imports CSV/Excel outputs with auto-mapping to the right sample IDs, and supports flexible workflows for everything from diagnostics to biobanking. As the UK LIMS market continues shifting toward automation and interoperability, Scispot offers a future-proof foundation—giving labs both the efficiency gains they expect today and the scalability required for tomorrow’s data-intensive quality control environment.

The Future of LIMS in Quality Control
As technology continues to advance, the role of LIMS in quality control is expected to grow. Innovations in data management software and laboratory automation will further enhance the capabilities of LIMS, making it an even more integral part of laboratory operations.
Integration with Other Lab Management Tools
Future developments in LIMS will likely focus on greater integration with other lab management tools and systems. This integration will enable seamless data exchange between different platforms, improving collaboration and communication within the laboratory.
Emphasis on Research Data Management
As research becomes more data-intensive, the need for effective research data management will increase. LIMS will play a crucial role in managing large datasets, ensuring that data is organized, accessible, and secure. This capability will be particularly important for laboratories engaged in cutting-edge research where data accuracy and integrity are paramount.

Conclusion
In conclusion, Laboratory Information Management Systems are vital for quality control. They reduce manual steps. They improve consistency. They help teams stay audit-ready.
If you want a system that goes beyond record keeping, Scispot brings LIMS workflows, integrations, and structured data into one place. It supports no-code configuration and instrument connectivity, so QC rules and results stay tied to the right sample IDs without constant spreadsheet stitching. That edge shows up in user feedback too. On G2, Scispot scores higher than LabWare LIMS for ease of setup (10.0 vs 7.9) and workflow management (10.0 vs 8.9). For UK labs working toward ISO 15189 expectations, this means cleaner traceability and stronger audit evidence, without adding more admin work.





.webp)
.webp)
.webp)



