Optimize Your Lab Audit Preparation: Key Steps
Preparing for a laboratory audit can seem daunting, but with the right planning and tools, the process becomes much more manageable. Meticulous attention to detail is key, as audits ensure compliance with industry standards and regulations. The right approach will not only ensure a smooth audit but also contribute to better overall lab efficiency.
Understanding Laboratory Audits: Purpose and Process
Laboratory audits are essential for maintaining the highest standards of quality and compliance. They assess how well your lab adheres to industry regulations, helping identify areas for improvement. Understanding the audit's purpose allows your lab to better prepare by aligning operations with regulatory requirements.

A well-executed audit involves a review of multiple areas:
- Quality control measures: Ensuring that tests meet accuracy and reliability standards.
- Equipment calibration and maintenance records: Verifying that instruments are consistently maintained and calibrated.
- Documentation and training records: Ensuring that all protocols are well-documented and staff are adequately trained.
Preparing your lab involves understanding the audit’s scope, ensuring your processes meet these requirements, and making any adjustments in advance to ensure compliance.
Audit Process Overview: What to Expect
A typical audit follows a structured process designed to review lab operations thoroughly. The process usually begins with a preliminary meeting, where auditors will outline objectives and gather essential information. They will then dive deeper into the audit by examining the following:
- Compliance with quality control standards.
- Verification of equipment calibration records.
- The organization and accessibility of documentation.
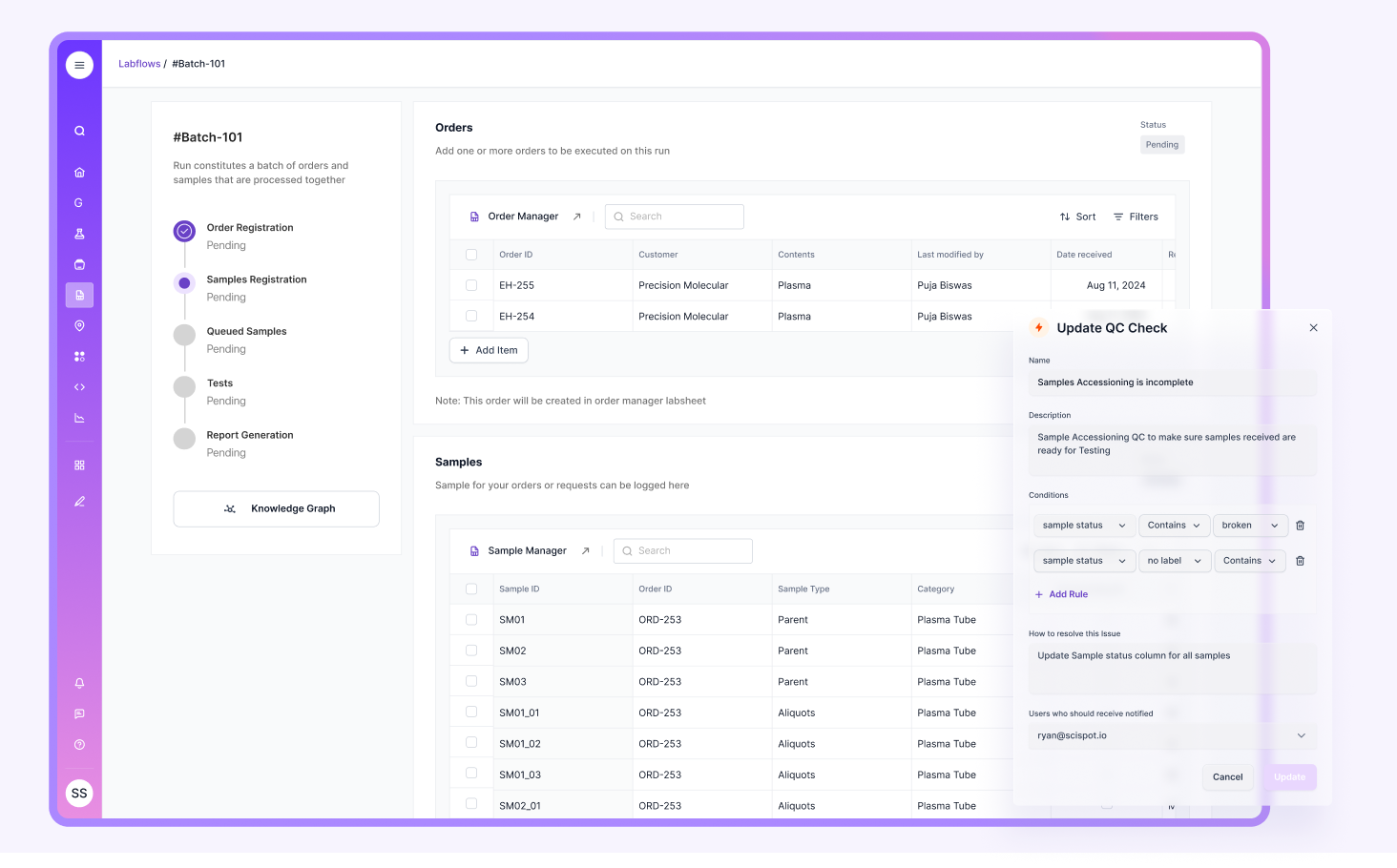
Anticipating these aspects can help minimize disruptions and streamline the audit process. With Scispot’s unified platform, all of your lab’s critical data—from test results to calibration records—are easily accessible, enabling a smoother audit experience.
Building a Lab Audit Checklist
A comprehensive checklist is vital for ensuring that all areas are addressed before the audit. It should cover every critical aspect of the lab’s operations and provide a structured approach to preparation. Here are some key areas to consider:
- Equipment calibration and maintenance: Ensure all calibration records are up to date.
- Quality control procedures: Review all protocols to ensure they are in compliance with industry standards.
- Documentation: Confirm that all SOPs, training records, and results are easily accessible.

Using Scispot's Labsheets and integrated tools, you can automate many of these tasks, ensuring that all documents are in order and easy to retrieve, reducing the likelihood of missing or outdated records during the audit.
Key Areas of Lab Quality Control to Review
Auditors will closely examine your lab’s quality control processes to assess the reliability and consistency of your results. Key areas include:
- SOPs: These should be regularly reviewed and updated to align with the latest standards.
- Data integrity: Verify that processes are in place to prevent errors in data handling.
- Instrument calibration: Ensure that instruments are calibrated regularly and the records are well-maintained.
Scispot helps streamline these processes by integrating quality control features directly into the workflow, making it easier to track and maintain compliance without extra administrative work.
How Scispot Enhances Audit Preparation and Compliance
Scispot is the ideal solution for labs preparing for audits, as it streamlines audit preparation, ensuring compliance with industry standards. By integrating LIMS, ELN, SDMS, and AI-driven solutions into one platform, Scispot provides a unified system for managing laboratory data, improving quality control, and ensuring traceability. With Scispot, labs can automate key audit tasks, such as generating accurate and up-to-date records for calibration, maintenance, and staff training, making the audit process smoother and more efficient. The platform's seamless integration capabilities allow for automated data flow, reducing the risk of errors and delays that typically arise during manual audits.
Moreover, Scispot's compliance-driven features, such as electronic signatures, audit trails, and role-based access control, ensure that all data remains secure and aligned with regulatory requirements. By providing a centralized hub for all laboratory operations, Scispot enhances your ability to quickly retrieve and organize critical documents and data, reducing the time spent preparing for audits. The result is not only a successful audit but also an ongoing culture of compliance, where labs can continuously monitor and improve their processes, thus staying ahead of industry standards.
Documentation and Record-Keeping Essentials
Well-organized, accessible documentation is vital for a successful audit. Ensure that all required records are not only accurate but easy to retrieve. Key documentation includes:
- Calibration and maintenance logs.
- Training certificates.
- SOPs.
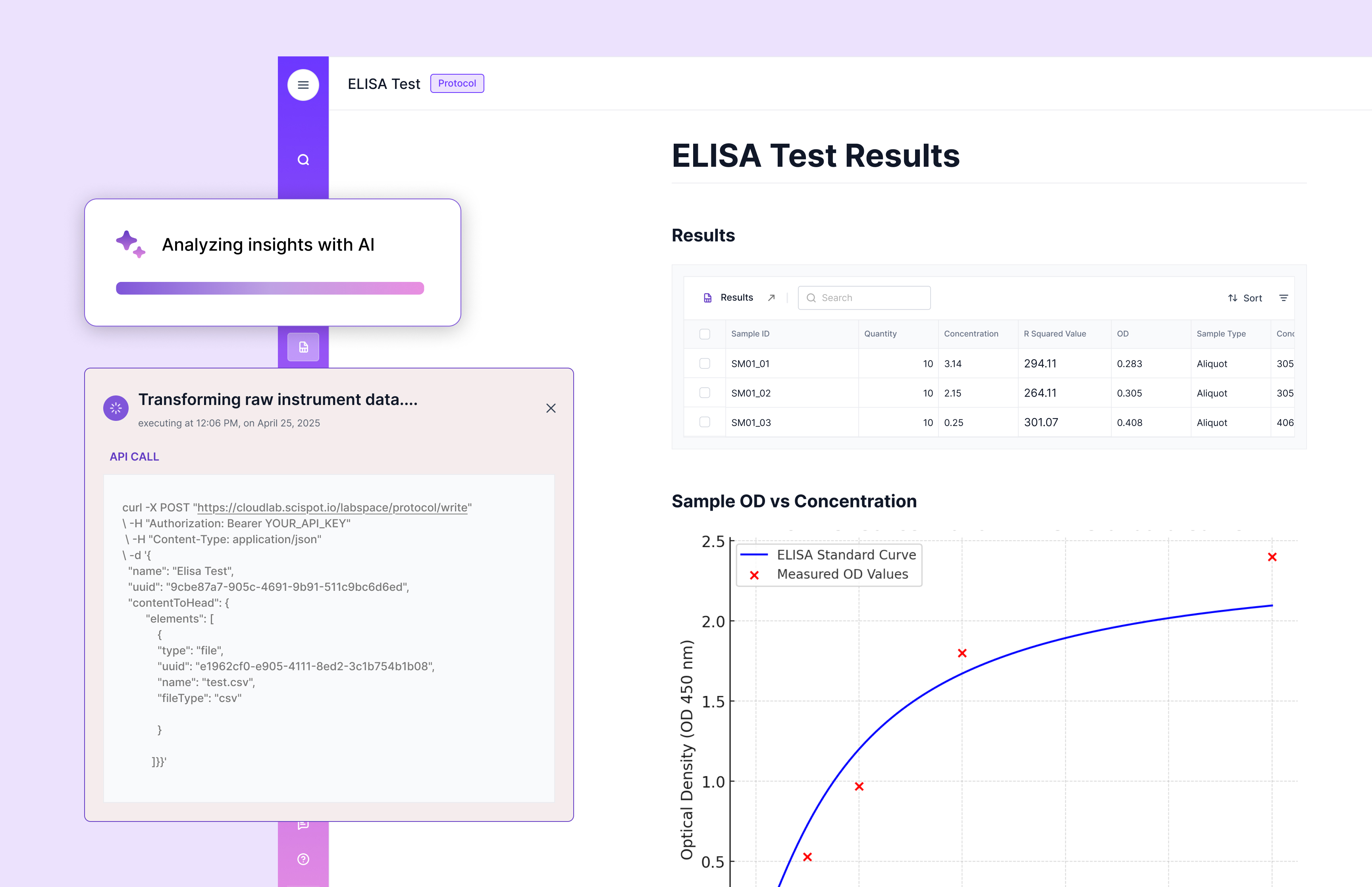
With Scispot, all of these records are stored in a central location, automatically updated, and accessible to auditors in real-time, eliminating the risk of misplaced or outdated documents.
Staff Training and Roles During the Audit
Training your team is crucial for a smooth audit process. Each member should be well-versed in their role and know how to respond to auditors' requests. This preparation will help ensure that the audit runs efficiently and confidently.
Focus on the following areas during staff training:
- Understanding the audit process.
- Knowing their specific responsibilities.
- Being able to access documents quickly.
Scispot’s Labsheet system offers real-time collaboration, ensuring that team members can access and update documents as needed, keeping everyone on the same page during the audit.

Audit Preparation Tips for a Smooth Process
Effective preparation is the key to a smooth audit. Here are a few practical tips to help streamline the process:
- Schedule internal audits to identify any weaknesses in your processes before the official audit.
- Use technology to improve document management and retrieval—Scispot’s platform offers this integration automatically.
- Engage a quality assurance consultant if needed to ensure compliance with all regulatory standards.
By leveraging Scispot’s integrated solution, your lab can minimize manual efforts, focus on continuous improvement, and stay audit-ready at all times.
After the Audit: Next Steps and Continuous Improvement
Once the audit concludes, it's essential to review the findings and act on any identified gaps. Scispot helps streamline this process by offering robust reporting and tracking features, allowing labs to easily monitor corrective actions and ensure continuous improvement.
.gif)
Incorporate feedback from the audit into your workflows, and ensure that any necessary changes are implemented promptly. Regularly assess lab processes and make adjustments to improve efficiency, compliance, and quality.
By adopting Scispot, labs can not only pass their audits with ease but also continuously improve their operations, ensuring long-term compliance and operational excellence.




.webp)
.webp)
.webp)
%20(1).webp)



